| 小胃肠间质瘤的CT增强扫描表现及漏误诊原因分析 |
2. 江苏省盱眙县人民医院影像科,江苏 盱眙 211700
胃肠间质瘤(gastrointestinal stromal tumors,GISTs)是胃肠道最常见的间叶源性肿瘤,通常认为其起源于胃肠道固有肌层Cajal细胞或其前体细胞,可发生于胃肠道的任何区域,也可见于网膜、肠系膜、腹膜后、盆腔等[1]。其确诊主要依靠病理学检查、免疫组织化学检查及基因检测。CD117、DOG1阳性支持诊断;CD117和/或DOG1阴性的疑似GISTs,KIT或PDGFRA激活突变的基因分析有助于确诊。小GISTs为直径<2 cm的GISTs[1],术前CT增强扫描易漏误诊。既往研究大多分析直径 > 2 cm的GISTs[2],而小GISTs CT增强扫描表现的相关研究较少。因此,笔者对小GISTs的CT增强扫描图像及临床资料进行分析,总结其CT增强扫描表现及漏误诊原因。
1 资料与方法 1.1 一般资料回顾性分析2018年1月至2021年3月在扬州大学附属苏北人民医院行手术治疗且术前行CT增强扫描的小GISTs患者56例,男23例,女33例;平均年龄(60.57±8.83)岁,其中男性(62.78±10.38)岁,女性(59.03±7.35)岁。
1.2 纳入及排除标准① 纳入标准:术后病理证实为GISTs;术前接受CT增强扫描;肿瘤长径<2 cm。②排除标准:GISTs术后转移或复发;因其他胃肠道肿瘤手术术中偶然发现者。
1.3 仪器与方法采用GE Optima CT660、Light SpeedVCT、Discovery CT750 HD、宝石能谱CT、联影UCT780等CT机,对患者行增强扫描。扫描前常规禁食8 h,扫描前1.5 h分3次饮2.0%泛影葡胺溶液1 500 mL以充盈肠道。扫描参数:120 kV,200 mA,层厚、层距均为5 mm。对动脉期、门静脉期图像分别行1 mm薄层重建及冠状位、矢状位重建。增强扫描经肘静脉注射对比剂碘海醇(碘浓度350 mg/mL),剂量80~100 mL,流率2.5~3.0 mL/s,于注射对比剂后25~30、60~70 s行动脉期和门静脉期扫描。
1.4 图像分析由3名高年资诊断医师在不知病理的情况下,对CT图像进行分析,统计胃肠道准备情况及影像表现。胃肠道准备情况包括检查前是否禁食、是否饮入泛影葡胺溶液及胃肠道是否充盈等;影像表现主要包括病灶部位、生长方式(腔内、腔外或腔内外)、大小(最大层面最长径)、形状(类圆形、不规则形)及强化表现等。肿瘤CT值的测量方法:测量面积3~10 mm2,测量3次,取平均值,测量时尽量避开肿瘤边缘、囊变区、钙化区及有伪影的区域。
1.5 统计学分析采用SPSS 25.0软件对数据进行分析。计量资料以x±s表示,2组间持续变量的比较采用t检验。2组计数资料的比较采用χ2检验或费希尔精确概率检验。以P<0.05为差异有统计学意义。
2 结果 2.1 一般资料56例中,位于胃49例,其中贲门区12例(图 1)、胃底21例(图 2,3)、胃体15例(图 4,5)、胃窦1例,小肠4例(图 6),结直肠1例(图 8),肛周1例(图 7),胃肠道外1例。传统开腹手术6例,腹腔镜手术9例,内镜下黏膜剥离术(ESD)26例,内镜下全层切除术(EFR)15例。56例核分裂象均≤5/50 HPF,CD34、DOG-1均呈阳性;SMA阳性4例,阴性52例。肿瘤危险度(NIH改良版):极低危55例,高危1例(瘤体破裂出血,图 8)。
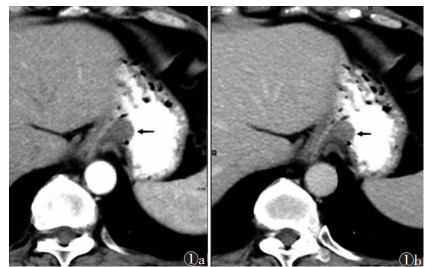 |
| 图 1 女,54岁,贲门下小胃肠间质瘤(GISTs),腔内型(黑箭) 图 1a CT增强扫描动脉期病灶CT值32 HU 图 1b 门静脉期CT值55 HU |
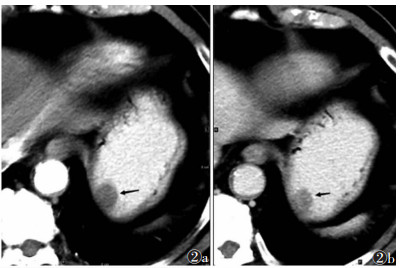 |
| 图 2 女,55岁,胃底小GISTs,腔内型(黑箭) 图 2a CT增强扫描动脉期病灶CT值46 HU 图 2b 门静脉期CT值约67 HU |
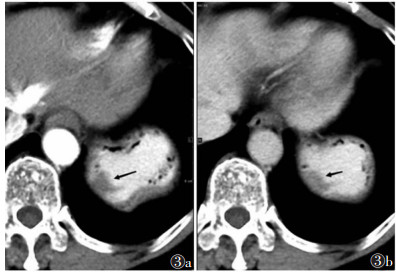 |
| 图 3 女,64岁,形态不规则的小胃肠间质瘤,腔内型(黑箭) 图 3a CT增强扫描动脉期病灶CT值44 HU 图 3b 门静脉期CT值86 HU |
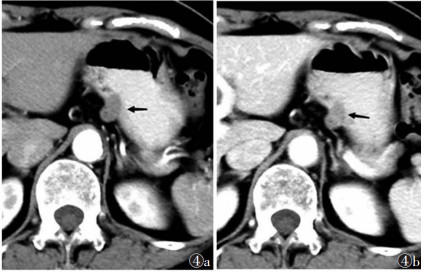 |
| 图 4 女,60岁,胃体小弯侧小GISTs,混合型(黑箭) 图 4a CT增强扫描动脉期病灶CT值75 HU 图 4b 门静脉期CT值90 HU |
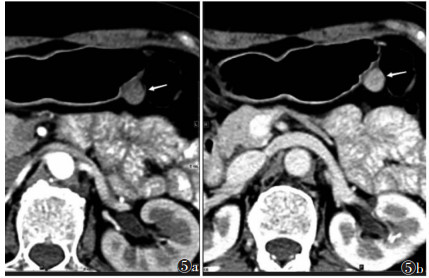 |
| 图 5 女,51岁,胃体小GISTs,腔外型(白箭) 图 5a CT增强扫描动脉期病灶CT值50 HU 图 5b 门静脉期CT值94 HU |
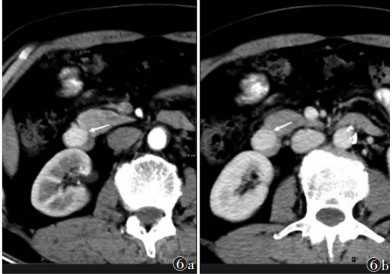 |
| 图 6 男,61 岁,十二指肠降段小 GISTs,腔内型(白箭) 图 6a CT 增强扫描动脉期病灶 CT 值 132 HU 图 6b 门静脉期 CT 值 130 HU |
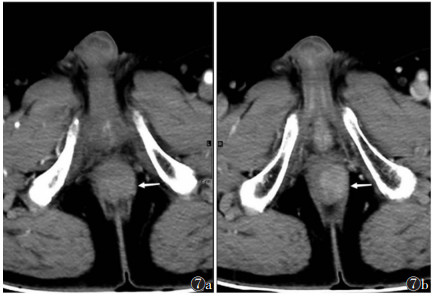 |
| 图 7 男,77岁,肛管左缘小GISTs,腔外型(白箭) 图 7a CT增强扫描动脉期瘤体的CT值61 HU 图 7b 门静脉期CT值96 HU |
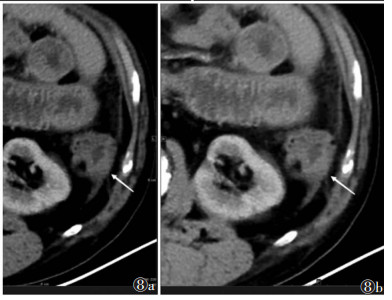 |
| 图 8 男,50岁,降结肠小GISTs破裂、出血,可见局部坏死区(白箭) 图 8a CT增强扫描动脉期实质部分CT值51 HU 图 8b 门静脉期CT值81 HU |
2.2 小GISTs的CT增强扫描表现
56例中,10例经3位诊断医师讨论研究后仍未能明确肿瘤位置,CT增强扫描明确显示46例。其中,类圆形36例,形态不规则10例;腔内型31例(67.39%),腔外型7例,混合型8例;7例伴钙化;强化不均匀6例,出现局部囊变坏死。46例瘤体实质部分均可见强化,瘤体实质部分动脉期、门静脉期的CT值分别为(55.70±19.16)、(76.28±19.46)HU,平均值差为(-20.59±13.88)HU,两者比较,差异有统计学意义(t=-10.060,P<0.001)。
2.3 小GISTs的漏误诊情况56例中,CT增强扫描漏诊19例(33.93%),其中9例(16.07%)经3位诊断医师讨论研究后明确肿瘤位置。本研究影像表现及误诊分析主要针对能明确显示肿瘤的46例CT增强扫描图像;漏诊分析则针对56例CT增强扫描图像。
46例中误诊10例(21.74%),其中误诊为神经鞘瘤2例、平滑肌瘤2例、异位胰腺1例、神经内分泌肿瘤1例、上皮源肿瘤2例、钙化灶2例(图 9)。
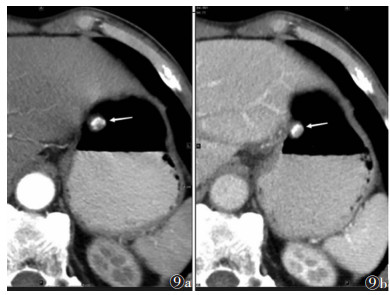 |
| 图 9 男,77岁,含有钙化灶的胃体小弯侧小GISTs,腔内型,边缘可见部分肿瘤实质(白箭) 图 9a CT增强扫描动脉期瘤体实质区CT值63 HU 图 9b 门静脉期CT值90 HU |
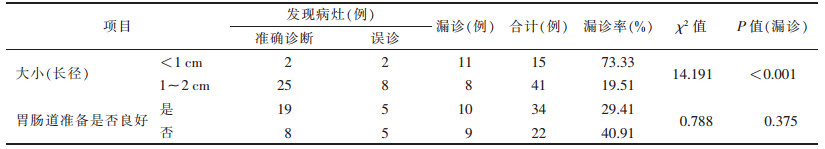
漏诊19例中,胃部漏诊17例,其中贲门区4例、胃底区8例、胃体区5例;小肠部位漏诊2例。不同肿瘤大小的漏诊率比较,差异有统计学意义(P<0.05);不同胃肠道准备情况的漏诊率比较,差异无统计学意义(P>0.05,表 1)。CT增强扫描5 mm层厚图像漏诊25例,发现病灶31例,漏诊率44.64%;薄层重建后图像漏诊19例,发现病灶37例,漏诊率33.93%,2种方法漏诊率比较,差异无统计学意义(χ2=1.348,P=0.246)。
| 表 1 不同肿瘤大小、胃肠准备情况的漏误诊情况(n=56) |
 |
3 讨论
近年来,无明显症状的小GISTs检出率明显提高。有报道发现,胃小GISTs在年龄 > 50岁的人群尸检中检出率高达22.5%[3]。本研究平均年龄(60.57±8.83)岁,女性(58.93%,33/56)多于男性(41.07%,23/56),与以往研究[4]相符。目前对小GISTs的诊疗尚无统一意见,有学者认为,随访可能是必要的[5],也有学者认为,小GISTs有一定的恶性潜能,非胃来源小GISTs的生物学行为更差,须考虑早期完整切除[6],手术通常根据肿瘤大小、位置、生长方式和肿瘤学团队进行选择。近年来,内镜下切除在小GISTs治疗中应用越来越广泛,本研究内镜下治疗(包括ESD、EFR)41例(73.21%)。内镜下治疗具有创伤小、恢复快等特点,可缩短患者住院时间、减轻经济负担;但存在出血、穿孔风险,能否达到显微镜下切缘无肿瘤组织残留(R0切除)也是应考虑的问题[7]。
超声内镜能够准确显示肿瘤起源、范围、局部脏器浸润情况等,诊断小GISTs具有独特优势[8]。近年来超声内镜应用广泛,但其对其他常见黏膜下肿物,如脂肪瘤、异位胰腺、平滑肌瘤和施万细胞瘤等的鉴别诊断有一定困难。而CT增强扫描可清晰显示肿瘤位置、形态、大小、与周围组织关系、强化表现等,在小GISTs的术前鉴别诊断及随访中具有重要意义[9]。另外,CT增强扫描方便、安全,患者依从性较好,适用于体质差、年龄大等不能耐受侵入性、有创性检查的GISTs患者。
本研究小GISTs多发生于胃(49例,87.50%),46例能在CT增强扫描明确显示,其中36例形态规则,呈类圆形,与高芙蓉等[10]的研究相符。7例伴钙化。瘤体实质部分均可见强化,动脉期平均CT值(55.70±19.16)HU,静脉期平均CT值(76.28±19.46)HU,平均差值(-20.59±13.88)HU,差异有统计学意义,与杨兴益等[11]研究结果基本一致。43例(93.48%)门静脉期强化程度高于动脉期,3例(胃、小肠、肛周各1例)增强扫描后动脉期及门静脉期均呈明显强化,门静脉期高于动脉期。
小GISTs的CT增强扫描表现易与胃肠道神经鞘瘤、神经内分泌肿瘤、异位胰腺及平滑肌瘤等混淆,应注意鉴别诊断。①He等[12]研究指出,GISTs与胃肠道神经鞘瘤大小、形状、是否囊变、肿瘤周围淋巴结、肿瘤血管、强化方式及程度的差异均有统计学意义,胃肠道神经鞘瘤通常表现为胃部圆形、均质、低密度病变[13],强化程度相对较低(图 10)。②大部分神经内分泌肿瘤的动脉早期强化更加明显,延迟期强化减低明显,一般低于胃肠间质瘤[14]。③异位胰腺通常表现为壁内椭圆形肿块,直径较小,且往腔内生长。以腺泡组织成分为主的异位胰腺在CT图像上呈均匀明显强化,其密度和强化模式与正常胰腺相似;而以导管为主的异位胰腺则呈不均匀轻度强化[15]。且异位胰腺多位于胃窦远端,长径与短径比值大,胃的异位胰腺在肿瘤与浆膜层之间可能会有脂肪间隙,而小GISTs在肿瘤与浆膜层之间存在脂肪间隙的可能性极低[16]。④胃肠道平滑肌瘤通常位于食管胃交界处,密度均匀,呈轻度强化,向管腔内生长[17]。当小GISTs瘤体大部分钙化时,可误诊为钙化灶。
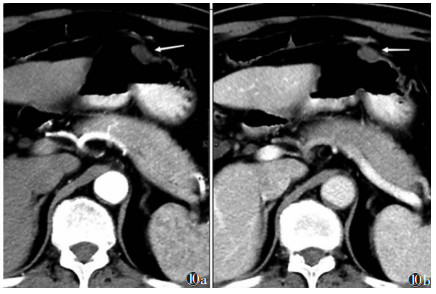 |
| 图 10 男,72岁,胃体部小神经鞘瘤,呈腔内生长(白箭) 图 10a CT增强扫描动脉期病灶CT值15 HU 图 10b 门静脉期CT值30 HU |
肿瘤大小及胃肠道准备不佳可能是漏诊的主要原因:本组长径<1 cm者,漏诊率73.33%(11/15);长径1~2 cm者漏诊率19.51%(8/41),两者比较差异有统计学意义(P<0.05)。本研究56例小GISTs中,胃肠道准备不佳者漏诊率40.91%(9/22),明显高于胃肠道准备较好者(29.41%,10/34),表明胃肠道准备在CT增强扫描中的重要性。
本研究中漏诊的小GISTs多位于胃底、贲门、胃体及小肠。原因可能是:①肿瘤位于胃与邻近脏器(包括膈肌、脾脏、肝脏)交界区,受呼吸伪影或容积效应的影响;部分可能形态不规则,呈匍匐生长,与胃黏膜层分界不清[18]。②部分腔内生长型的小GISTs,可能会因阳性对比剂的影响,而显示不清[5]。③小肠迂曲细长,不易观察可能会增加漏诊的可能性。本研究薄层重建图像的漏诊率(33.93%)低于5 mm层厚CT增强扫描图像的漏诊率(44.64%);且薄层及三维重建后,CT增强扫描中小GISTs的肿瘤形态、边界、密度显示更清晰。
本研究存在的不足:①为单中心研究,样本量较小,统计学结果可能存在偏差,相关结论还需大样本的研究证实。②本研究均为小GISTs的CT增强扫描图像,未对其他黏膜下肿瘤进行对比,其影像学差异的科学性需进一步分析。③患者仅行CT增强扫描,未将CT增强扫描的CT值与平扫CT值进行比较分析。
综上所述,小GISTs多位于胃及小肠,以腔内型多见;大多形态规则,呈类圆形,可出现局部钙化、囊变坏死;CT增强扫描后实质区均可见强化,且多呈渐进性强化(门静脉期强化高于动脉期)。长径<1 cm的小GISTs较长径为1~2 cm者在CT增强扫描中更易漏诊。腹部CT检查时,应尽量做好胃肠道准备,以降低小GISTs的漏诊率;薄层及三维重建可能更有利于病灶的检出。应充分掌握小GISTs的CT增强扫描征象,减少小GISTs的误诊、漏诊。
| [1] |
VON MEHREN M, RANDALL R L, BENJAMIN R S, et al. Soft tissue sarcoma, version 2.2018, NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw, 2018, 16(5): 536-563. DOI:10.6004/jnccn.2018.0025 |
| [2] |
李玉舟, 金红瑞, 李春荣, 等. 34例胃肠间质瘤患者128层螺旋CT影像表现特点及诊断价值[J]. 中国CT和MRI杂志, 2017, 15(11): 102-105. |
| [3] |
AGAIMY A, WUNSCH P H, HOFSTAEDTER F, et al. Minute gastric sclerosing stromal tumors(GIST tumorlets) are common in adults and frequently show c-KIT mutations[J]. Am J Surg Pathol, 2007, 31(1): 113-120. DOI:10.1097/01.pas.0000213307.05811.f0 |
| [4] |
JOENSUU H, HOHENBERGER P, CORLESS C L. Gastrointestinal stromal tumour[J]. Lancet, 2013, 382(9896): 973-983. DOI:10.1016/S0140-6736(13)60106-3 |
| [5] |
NISHIDA T, GOTO O, RAUT C P, et al. Diagnostic and treatment strategy for small gastrointestinal stromal tumors[J]. Cancer, 2016, 122(20): 3110-3118. DOI:10.1002/cncr.30239 |
| [6] |
GIULIANO K, EJAZ A, REAMES B N, et al. Comparing the long-term outcomes among patients with stomach and small intestine gastrointestinal stromal tumors: an analysis of the national cancer database[J]. J Surg Oncol, 2018, 118(3): 486-492. |
| [7] |
LIU Z, ZENG Z, OUYANG S, et al. Comparison among endoscopic, laparoscopic, and open resection for relatively small gastric gastrointestinal stromal tumors (< 5 cm): a bayesian network metaanalysis[J]. Front Oncol, 2021, 11: 672364. DOI:10.3389/fonc.2021.672364 |
| [8] |
叶颖江, 沈琳, 李健, 等. 小胃肠间质瘤诊疗中国专家共识(2020年版)[J]. 临床肿瘤学杂志, 2020, 25(4): 349-355. DOI:10.3969/j.issn.1009-0460.2020.04.012 |
| [9] |
NISHIDA T, GOTO O, RAUT C P, et al. Diagnostic and treatment strategy for small gastrointestinal stromal tumors[J]. Cancer, 2016, 122(20): 3110-3118. DOI:10.1002/cncr.30239 |
| [10] |
高芙蓉, 卞巍, 俞丽. 胃肠道间质瘤的MSCT表现[J]. 医学影像学杂志, 2018, 28(3): 422-425. |
| [11] |
杨兴益, 李朝军, 郭浩. 多层螺旋CT联合超声内镜在胃肠道间质瘤诊断及预后评估中的临床价值[J]. 中国CT和MRI杂志, 2019, 17(10): 117-119. |
| [12] |
HE M Y, ZHANG R, PENG Z, et al. Differentiation between gastrointestinal schwannomas and gastrointestinal stromal tumorsby computed tomography[J]. Oncol Lett, 2017, 13(5): 3746-3752. DOI:10.3892/ol.2017.5955 |
| [13] |
XU J X, YU J N, WANG X J, et al. A radiologic diagnostic scoring model based on CT features for differentiating gastric schwannoma from gastric gastrointestinal stromal tumors[J]. Am J Cancer Res, 2022, 12(1): 303-314. |
| [14] |
REN S, CHEN X, WANG J, et al. Differentiation of duodenal gastrointestinal stromal tumors from hypervascular pancreatic neuroendocrine tumors in the pancreatic head using contrastenhanced computed tomography[J]. Abdom Radiol(NY), 2019, 44(3): 867-876. DOI:10.1007/s00261-018-1803-x |
| [15] |
YANG C W, CHE F, LIU X J, et al. Insight into gastrointestinal heterotopic pancreas: imaging evaluation and differential diagnosis[J]. Insights Imaging, 2021, 12(1): 144. DOI:10.1186/s13244-021-01089-0 |
| [16] |
LIU C, YANG F, ZHANG W, et al. CT differentiation of gastric ectopic pancreas from gastric stromal tumor[J]. BMC Gastroenterol, 2021, 21(1): 52. DOI:10.1186/s12876-021-01617-8 |
| [17] |
LEE M J, LIM J S, KWON J E, et al. Gastric true leiomyoma: computed tomographic findings and pathological correlation[J]. J Comput Assist Tomogr, 2007, 31(2): 204-208. DOI:10.1097/01.rct.0000237812.95875.bd |
| [18] |
李文平, 郭勇, 胡莹莹, 等. 口服对比剂CT全胃肠道造影方法评估[J]. 实用放射学杂志, 2018, 34(11): 1769-1772. |
 2022, Vol. 20
2022, Vol. 20


