2. 山东省海洋资源与环境研究院,山东省海洋生态修复重点实验室,山东 烟台 264006
阿特拉津,作为一种三嗪类除草剂,因其低成本和出色的除草效果,已在全世界范围内广泛使用[1]。2018年,阿特拉津的全球销售额高达6.55亿美元,占三嗪类除草剂市场份额的46.8%[2],在中国农业上的年使用量约为1 000~1 500 t[3]。尽管其效果显著,但阿特拉津在土壤中的半衰期较长,约为150~360 d,这意味着它有可能长期存在并污染我们的环境。更糟糕的是,这种化学物质在施用后会进入河流、湖泊等地表水,最终汇入海洋,成为海洋环境中的常见污染物[4]。在中国海州湾和澳大利亚大堡礁海水中,阿特拉津的浓度分别达到了61.9和730 ng/L[5-6]。另外,在中国海参样品中也检测到了阿特拉津,含量为0.9~3.62 μg/kg[7]。
随着阿特拉津在海洋环境与生物体内不断被检出,它对海洋生态系统的潜在危害引起了人们的持续关注,这种化学物质不仅对海洋生物产生直接毒性效应,而且通过食物链累积,对更高营养级的生物产生影响。例如:将三角褐指藻(Phaeodactylum tricornutum)暴露于3.2 μg/L阿特拉津7 d后,其叶绿素a含量显著降低,细胞结构受到破坏,种群生长受到抑制[8];Bejarano等[9]发现,将3种常见桡足类(Enhydrosoma baruchi, Onychocamptus sp.和Paronychocamptus wilsoni)暴露于26 μg/L阿特拉津28 d后,其丰度明显降低,进而改变了底栖动物的群落结构。另有研究报道,将大西洋鲑鱼(Salmo salar)暴露于100 μg/L阿特拉津10 d后,出现了离子调节、生长和内分泌紊乱的情况[10]。鉴于阿特拉津对海洋生态系统的潜在危害,制定相应的海水水质基准(Water quality criteria, WQC)显得尤为重要。WQC指水环境中的污染物或有害因素对人体健康或水生态系统不产生有害影响的最大浓度[11]。WQC的推导方法有评估因子法(Assessment factor, AF)、物种敏感度排序法(Species sensitivity rank, SSR)和物种敏感度分布法(Species sensitivity distribution, SSD)等[12]。Chen等[13]利用AF法,将绿藻的半数效应浓度(Median effect concentration, EC50)除以100,得到阿特拉津的预测无效应浓度(Predicted no effect concentration, PNEC)为1 050 ng/L;Papadakis等[14]同样利用AF法将藻类的无观察效应浓度(No observed effect concentration, NOEC)除以10,得到阿特拉津的PNEC为10 000 ng/L。Caquet等[15]使用SSD法拟合阿特拉津对藻类的NOEC值,推导出5%物种危害浓度(Hazardous concentration for 5% of the species, HC5)值为2 200 ng/L。以上这些研究中所用的生物毒性数据较少,且主要基于单一生物类群的数据,难以为制订阿特拉津的海水WQC提供有力支撑。
为制定阿特拉津的海水WQC提供有力支撑,本研究搜集和筛选了阿特拉津对海洋生物的急性和慢性毒性数据,利用SSD法推导了阿特拉津的长期和短期海水WQC。同时,还调查了莱州湾海水中阿特拉津的污染现状,并利用本研究推导的长期海水WQC评估了阿特拉津在该海域中的生态风险。
1 材料与方法 1.1 阿特拉津阿特拉津,化学名称为2-氯-4-乙胺基-6-异丙胺基-1,3,5-三嗪,分子式为C8H14ClN5。其在海水中的半衰期约为27.7 d,主要降解产物为去乙基阿特拉津与去异丙基阿特拉津[16]。
1.2 毒性数据的搜集与筛选本研究从美国环保署(US EPA)的生态毒理数据库(https://cfpub.epa.gov/ecotox/)、Web of Science (http://apps.webofknowledge.com)和中国知网(http://www.cnki.net/)等数据库中搜集已发表的阿特拉津毒性数据,时间截至2022年1月1日。根据《HJ 1260—2022海洋生物水质基准推导技术指南(试行)》[11]和“澳大利亚和新西兰水质基准制定的指导方针”[17]对数据进行筛选。对于急性毒性数据,暴露时间以1~4 d为宜;对于慢性毒性数据,微藻的暴露时间应大于1 d,小型无脊椎动物和水生维管束植物的暴露时间应不少于7 d,大型无脊椎动物的暴露时间不少于14 d,鱼类和两栖类的幼体和成体的暴露时间应分别不少于7和21 d。搜集的毒性数据应至少涵盖3个营养级,至少包括10个物种,且涵盖以下生物类群:1种鱼科、2种甲壳科、1种非鱼类的底栖动物、1种浮游植物和1种水生维管束植物。当同一物种存在多个毒性数据时,取其几何平均值作为该物种最终的种平均急性和慢性毒性值。
1.3 水质基准推导利用搜集的毒性数据,以物种的毒性数据为x轴,以物种毒性数据的累积概率为y轴,构建SSD曲线。通过计算得到HC5值,再将HC5除以一个评估因子(取2)得到水质基准[18]。使用正态分布(Normal)、对数正态分布(Log-Normal)、对数逻辑斯蒂分布(Log-Logistic)和Bull Ⅲ分布等常用模型进行数据拟合。采用基于R语言的SSD Tools软件进行数据的拟合。
1.4 莱州湾海水样品采集如图 1和表 1所示,选取莱州湾作为调查海域,共设置了14个站位,于2021年3月进行采样。使用有机玻璃采水器采取表层(水深50 m以上)海水,通过GF/F玻璃微纤维滤膜(孔径0.22 μm,直径47 mm)进行过滤后运回实验室,以用于阿特拉津的提取。

|
图 1 莱州湾取样站位 Fig. 1 Sampling stations in Laizhou Bay |
|
|
表 1 莱州湾各站位坐标以及阿特拉津浓度 Table 1 Coordinates of sampling stations and concentrations of atrazine in the seawater |
将400 mL过滤后的表层海水与10 μL同位素内标母液(阿特拉津D5,100 μg/L)混匀后,利用预先活化的固相萃取柱(Oasis HLB)进行抽滤富集,用5 mL甲醇进行洗脱,将洗脱液收集在玻璃管中。然后将玻璃管中的洗脱液进行氮吹干燥,用复溶液(乙腈∶水=1∶9)进行复溶后,利用1 mL注射器将复溶液移出并过滤至色谱瓶中。根据Mazzella等[19]的方法,使用高效液相色谱-电喷雾质谱(HPLC-ESI-MS/MS)对阿特拉津进行测定。为验证方法的可靠性,设置了3个空白水样进行潜在污染分析,均未检测出阿特拉津。在固相萃取之前加入标准品测定回收率,监测样品预处理与仪器分析过程中的误差。本研究中阿特拉津的检出限为3 ng/L,回收率为96%,相对标准偏差为12%。
1.6 生态风险评估 1.6.1 商值法商值法的公式为
| $ R_{\mathrm{HQ}}=A_{\mathrm{EC}} / B_{\mathrm{WQC}}。$ |
式中:RHQ代表阿特拉津环境浓度与水质基准的比值;AEC代表阿特拉津在环境中的浓度;BWQC代表水质基准。根据RHQ的大小可以将风险水平分为四级:RHQ < 0.1表明阿特拉津的生态风险可以接受;0.1≤RHQ < 1表明存在低风险;1≤RHQ < 10表明存在中等风险;RHQ≥10表明存在高度风险[20-21]。
1.6.2 联合概率曲线(JPC)法本文使用风险评估软件BMC-SSD构建联合概率曲线(Joint probability curve, JPC)[22-24]。JPC是在得到毒性数据SSD曲线和阿特拉津环境浓度累积频率分布的基础上,以毒性数据SSD曲线的纵坐标为JPC的横轴,以超过毒性数值的环境浓度百分比为纵轴,形成累积剖面图,JPC与坐标轴之间的面积即为预期的生态风险[25]。
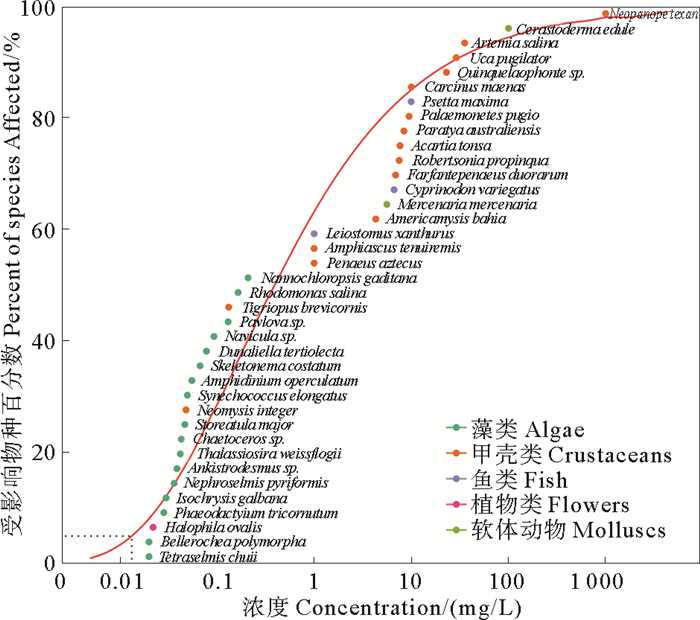
2 结果 2.1 阿特拉津的毒性数据本研究共搜集到阿特拉津对海洋生物的急性毒性数据76个,涵盖10门34科,共38个物种,如表 2所示。急性毒性数据从0.020 mg/L (扁藻(Tetraselmischuii)和鼓藻(Bellerochea polymorpha), EC50)到1 000 000 mg/L(扇蟹(Neopanopetexana), LC50) 不等。最敏感的物种与最不敏感的物种相差很大(相差约50 000倍)。在种平均急性毒性数据中,藻类和甲壳类数据最多,分别占比为44.7%和39.5%。
|
|
表 2 阿特拉津对海洋生物的急性毒性数据 Table 2 Acute toxicity data of atrazine to marine organisms |
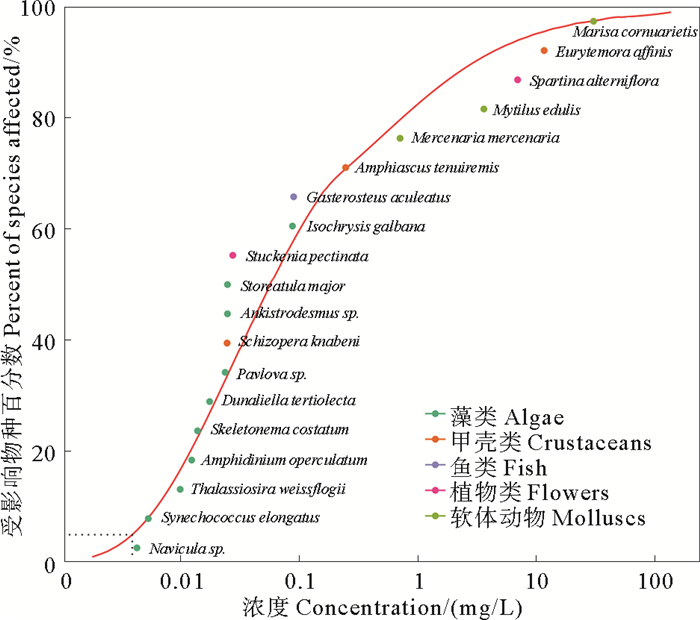
本研究共搜集到阿特拉津对海洋生物的慢性毒性数据32个,涵盖10门18科,共19个物种,如表 3所示。慢性毒性数据从0.004 mg/L(舟形藻(Navicula sp.), LOEC)到30.000 mg/L (月光螺(Marisa cornuarietis), NOEC) 不等。最敏感的物种与最不敏感的物种相差约6 900倍。在种平均慢性毒性数据中,藻类、甲壳动物和软体动物数据最多,分别占比为52.6%、15.8%和15.8%。
|
|
表 3 阿特拉津对海洋生物的慢性毒性数据 Table 3 Chronic toxicity data of atrazine to marine organisms |
使用5种模型对阿特拉津的急性和慢性数据进行拟合,结果表明Anderson-Darling(AD)和Kolmogorov-Smirnov(KS)检验均为P>0.05。AIC和AICC检验结果接近,其中Log-Gumbel模型的AICC值最小,参数Delta为0,如表 4所示。因此,本研究选择Log-Gumbel模型对阿特拉津的急性和慢性毒性数据拟合SSD曲线,得到HC5值分别为13 200 ng/L (见图 2)和3 950 ng/L (见图 3)。通过HC5值除以评估因子,得到阿特拉津的短期和长期海水水生生物水质基准,分别为6 600和1 975 ng/L。
|
|
表 4 阿特拉津海水急性和慢性毒性数据的五种拟合模型相关参数 Table 4 Five fitting model parameters for acute and chronic toxicity data of Atrazine |
|
图 2 阿特拉津对海洋生物的急性毒性数据SSD拟合曲线 Fig. 2 SSD fitting curve of acute toxicity data of atrazine to marine organisms |
|
图 3 阿特拉津对海洋生物的慢性毒性数据SSD拟合曲线 Fig. 3 SSD fitting curve of chronic toxicity data of atrazine to marine organisms |
在莱州湾海域的14个站位的表层海水中,阿特拉津的检出率为100%,浓度从48.15 ng/L到118.24 ng/L不等(见表 1)。从整体上看,阿特拉津的浓度呈现出由南部沿岸向北部海域递减的趋势(见图 4), 其中,站位B8和B6的阿特拉津浓度最高,分别为118.24和104.71 ng/L,站位B8的阿特拉津浓度分别是站位B3和B4的2.46和1.81倍。

|
图 4 阿特拉津在莱州湾的空间分布 Fig. 4 Spatial distribution of atrazine in Laizhou Bay |
基于本文推导的长期海水水质基准(1 975 ng/L),对阿特拉津浓度最高的站位B8进行风险评估,通过商值法得到RHQ为0.06(< 0.1)。结合莱州湾14个站位检测到的阿特拉津浓度,使用BMC-SSD软件导出联合概率曲线。如图 5所示,阿特拉津在莱州湾表层海水中的总体风险概率中值和均值分别为1.04%和0.79%。

|
图 5 莱州湾表层海水中阿特拉津的联合概率曲线 Fig. 5 Joint probability curves of atrazine in surface waters of Laizhou Bay |
本研究共获得阿特拉津对38种海洋生物的平均急性毒性数据,发现绿藻门扁藻(Tetraselmis chuii)和褐藻门鼓藻(Bellerochea polymorpha)对阿特拉津最为敏感,平均急性毒性值为0.02 mg/L。相比之下,节肢动物门的扇蟹(Neopanope texana)对阿特拉津最不敏感,平均急性毒性值达到1 000 mg/L。同时,慢性毒性数据显示褐藻门舟形藻(Navicula sp.)对阿特拉津最敏感,而软体动物门的月光螺(Marisa cornuarietis)则最不敏感。有研究表明,具膜舟形藻(Navicula pelliculosa)对与阿特拉津同为三嗪类除草剂的扑草净非常敏感,慢性毒性值仅为300 ng/L[63],这与本文得到的舟形藻对阿特拉津慢性毒性最敏感的结果一致。阿特拉津主要通过破坏植物光合系统Ⅱ (PS Ⅱ)反应中心来抑制受体和供体的电子传递[64]。浮游植物作为海洋初级生产者,通过光合作用固碳合成糖类、脂质和蛋白质等生物大分子,构成了海洋生态系统和海洋食物网初级生产力的基础[34]。在本研究中发现,浮游植物对阿特拉津最为敏感,因此特别提示应关注三嗪类除草剂对海洋初级生产力的影响。
SSD法是中国、荷兰、澳大利亚和新西兰等国家规定使用的水质基准推导方法。本研究利用5种模型拟合了阿特拉津的急性和慢性毒性数据,其中Log-Gumbel模型对数据的拟合最好,这与秦璐等[65]使用SSD法拟合除草剂高效氟吡甲禾灵的毒性数据时优选的模型一致。本研究推导阿特拉津的长期海水水质基准为1 975 ng/L,与加拿大保护水生生物的阿特拉津水质基准和欧盟规定的阿特拉津最大容许浓度(分别为1.8和2.0 μg/L)相近[5]。Moore等[66]使用SSD法拟合阿特拉津对水生自养生物的EC50值,推导出阿特拉津的HC5值为28.4 μg/L,明显高于本研究推导的水质基准。这可能是因为前者没有区分海水与淡水生物。考虑到盐度会对污染物的毒性具有明显影响,在推导海水水质基准时应该选择海洋生物毒性数据[17]。有研究表明,1 μg/L阿特拉津对大叶藻(Zostera marina L.)、丛生大叶藻(Z. caespitosa M.)和红须根虾形藻(Phyllospadix iwatensis M.)的光合作用未产生显著影响,而暴露浓度达到5 μg/L时,对3种海草的光合作用抑制率为6.89%~8.94%[67]。另有研究表明,将海葵(Exaiptasia diaphana)暴露于3 μg/L阿特拉津2周后,而其谷氨酸水平得到显著上调[68]。此外,本研究搜集到的阿特拉津对海洋生物的最低观察效应浓度(Lowest observed effect concentration, LOEC)均高于推导的阿特拉津海水水质基准。可见,本研究推导的阿特拉津海水水生生物水质基准可为海洋生物提供有效保护,因而可为制订海洋中阿特拉津的WQC提供科学依据。
本研究中,阿特拉津在莱州湾海域的浓度范围为48.15~118.24 ng/L,这与徐英江等[69]报道的浓度(6.82~83 ng/L)接近。对中国大连海域和澳大利亚大堡礁海域的多项研究发现,除草剂在近岸浓度较高,特别是河口区的阿特拉津浓度可达到0.4~3 μg/L[5, 70]。在本次阿特拉津调查中,莱州湾南部的B8站位浓度最高(118.24 ng/L),这可能是由莱州湾南部有较多河流汇入莱州湾海域所致,如胶莱河、潍河、白浪河和小清河等,这些河流沿岸是重要的农业区,可能存在较高的阿特拉津残留。2种风险评估结果都表明,莱州湾海域的阿特拉津尚未造成明显的影响,风险可以忽略。然而,值得注意的是,莱州湾有多条河流汇入,这些河流可能携带阿特拉津。在复杂的近海河口环境中,阿特拉津浓度受季节影响较大,因此需要在时间和空间维度上进一步调查阿特拉津的污染状况。
4 结论(1) 阿特拉津的短期和长期海水水质基准分别为6 600和1 975 ng/L。
(2) 阿特拉津在莱州湾表层海水中的浓度范围为48.15~118.24 ng/L,并且在南部河口沿岸海域污染水平最高。
(3) 莱州湾海水中阿特拉津的生态风险处于可接受水平,但仍需要关注汛期入海河流排放对阿特拉津污染的影响。
| [1] |
王北南, 宋晓, 贺琳娟, 等. 莠去津和芴对斑马鱼胚胎的联合毒性效应研究[J]. 农业环境科学学报, 2021, 40(10): 2086-2094. Wang N B, Song X, He L J, et al. Combined toxic effects of atrazine and fluorene on zebrafish embryos[J]. Journal of Agriculture Environment Science, 2021, 40(10): 2086-2094. DOI:10.11654/jaes.2021-0261 (  0) 0) |
| [2] |
顾林玲. 三嗪类除草剂研究与开发新进展[J]. 世界农药, 2021, 43(12): 12-23. Gu L L. New progress in research and development of triazine herbicides[J]. The World Pesticide, 2021, 43(12): 12-23. (  0) 0) |
| [3] |
刘勇攀, 张衿潇, 蒋燕虹, 等. 功能化材料Zr@AC对水中难降解农药阿特拉津的去除特征[J]. 环境科学研究, 2022, 35(3): 750-760. Liu Y P, Zhang J X, Jiang Y H, et al. Removal characteristics of the refractory pesticide atrazine in water by functionalized material Zr@AC[J]. Environmental Science Research, 2022, 35(3): 750-760. (  0) 0) |
| [4] |
Liu Y, Fan X, Zhang T, et al. Effects of the long-term application of atrazine on soil enzyme activity and bacterial community structure in farmlands in China[J]. Environmental Pollution, 2020, 262: 114264. DOI:10.1016/j.envpol.2020.114264 (  0) 0) |
| [5] |
Lewis S E, Brodie J E, Bainbridge Z T, et al. Herbicides: A new threat to the Great Barrier Reef[J]. Environmental Pollution, 2009, 157(8-9): 2470-2484. DOI:10.1016/j.envpol.2009.03.006 (  0) 0) |
| [6] |
张望, 范广宇, 孟祥龙, 等. 海州湾沿岸海水中21种除草剂的分布特征[J]. 江苏农业科学, 2019, 47(23): 289-294. Zhang W, Fan G Y, Meng X L, et al. Distribution characteristics of 21 herbicides in coastal seawater of Haizhou Bay[J]. Jiangsu Agricultural Sciences, 2019, 47(23): 289-294. (  0) 0) |
| [7] |
张华威, 刘慧慧, 田秀慧, 等. 凝胶色谱-固相萃取-气相色谱-串联质谱法测定水产品中9种三嗪类除草剂[J]. 质谱学报, 2015, 36(2): 177-184. Zhang H W, Liu H H, Tian X H, et al. Determination of nine triazine herbicides in aquatic products by gel chromatography-solid phase extraction-gas chromatography-tandem mass spectrometry[J]. Journal of Mass Spectrometry, 2015, 36(2): 177-184. (  0) 0) |
| [8] |
Yang L, Li H, Zhang Y, et al. Environmental risk assessment of triazine herbicides in the Bohai Sea and the Yellow Sea and their toxicity to phytoplankton at environmental concentrations[J]. Environment International, 2019, 133: 105175. DOI:10.1016/j.envint.2019.105175 (  0) 0) |
| [9] |
Bejarano A C, Pennington P L, DeLorenzo M E, et al. Atrazine effects on meiobenthic assemblages of a modular estuarine mesocosm[J]. Marine Pollution Bulletin, 2005, 50(11): 1398-1404. DOI:10.1016/j.marpolbul.2005.06.012 (  0) 0) |
| [10] |
Nieves-Puigdoller K, Björnsson B T, McCormick S D. Effects of hexazinone and atrazine on the physiology and endocrinology of smolt development in Atlantic salmon[J]. Aquatic Toxicology, 2007, 84(1): 27-37. DOI:10.1016/j.aquatox.2007.05.011 (  0) 0) |
| [11] |
中华人民共和国生态环境部. HJ 1260—2022海洋生物水质基准推导技术指南(试行)[S]. 北京: 环境科学出版社, 2022. Ministry of Ecology and Environment of the People's Republic of China. HJ 1260—2022 Technical Guideline for Deriving Water Quality Criteria for Marine Organisms(on Trial)[S]. Beijing: Environmental Science Press, 2022. (  0) 0) |
| [12] |
解瑞丽, 周启星. 国外水质基准方法体系研究与展望[J]. 世界科技研究与发展, 2012, 34(6): 939-944. Xie R L, Zhou Q X. Research and prospect of water quality criteria method system abroad[J]. World Scientific and Technological Research and Development, 2012, 34(6): 939-944. DOI:10.3969/j.issn.1006-6055.2012.06.018 (  0) 0) |
| [13] |
Chen C, Zou W, Cui G, et al. Ecological risk assessment of current-use pesticides in an aquatic system of Shanghai, China[J]. Chemosphere, 2020, 257: 127222. DOI:10.1016/j.chemosphere.2020.127222 (  0) 0) |
| [14] |
Papadakis E N, Tsaboula A, Kotopoulou A, et al. Pesticides in the surface waters of Lake Vistonis Basin, Greece: Occurrence and environmental risk assessment[J]. Science of the Total Environment, 2015, 536: 793-802. DOI:10.1016/j.scitotenv.2015.07.099 (  0) 0) |
| [15] |
Caquet T, Roucaute M, Mazzella N, et al. Risk assessment of herbicides and booster biocides along estuarine continuums in the Bay of Vilaine area (Brittany, France)RR[J]. Environmental Science and Pollution Research International, 2013, 20(2): 651-666. DOI:10.1007/s11356-012-1171-y (  0) 0) |
| [16] |
Yang L, Zhang Y. Effects of atrazine and its two major derivatives on the photosynthetic physiology and carbon sequestration potential of a marine diatom[J]. Ecotoxicology and Environmental Safety, 2020, 205: 111359. DOI:10.1016/j.ecoenv.2020.111359 (  0) 0) |
| [17] |
Australian and New Zealand Environment and Conservation Council, Agriculture and Resource Management Council of Australia and New Zealand. Water Quality Monitoring Guidelines[M]. Artarmon, New South Wales: Australian Water Association, 2000.
(  0) 0) |
| [18] |
Wang S, Wang J, Zhang X, et al. Freshwater quality criteria of four strobilurin fungicides: Interspecies correlation and toxic mechanism[J]. Chemosphere, 2021, 284: 131340. DOI:10.1016/j.chemosphere.2021.131340 (  0) 0) |
| [19] |
Mazzella N, Delmas F, Delest B, et al. Investigation of the matrix effects on a HPLC-ESI-MS/MS method and application for monitoring triazine, phenylurea and chloroacetanilide concentrations in fresh and estuarine waters[J]. Journal of Environmental Monitoring, 2009, 11(1): 108-15. DOI:10.1039/B805160G (  0) 0) |
| [20] |
Ding T, Du S, Zhang Y, et al. Hardness-dependent water quality criteria for cadmium and an ecological risk assessment of the Shaying River Basin, China[J]. Ecotoxicology and Environmental Safety, 2020, 198: 110666. DOI:10.1016/j.ecoenv.2020.110666 (  0) 0) |
| [21] |
Li L, Liu D, Zhang Q, et al. Occurrence and ecological risk assessment of selected antibiotics in the freshwater lakes along the middle and lower reaches of Yangtze River Basin[J]. Journal of Environmental Management, 2019, 249: 109396. DOI:10.1016/j.jenvman.2019.109396 (  0) 0) |
| [22] |
He W, Kong X, Qin N, et al. Combining species sensitivity distribution (SSD) model and thermodynamic index (exergy) for system-level ecological risk assessment of contaminates in aquatic ecosystems[J]. Environment International, 2019, 133(PtB): 105275. (  0) 0) |
| [23] |
He W, Qin N, Kong X, et al. Ecological risk assessment and priority setting for typical toxic pollutants in the water from Beijing-Tianjin-Bohai area using Bayesian matbugs calculator (BMC)[J]. Ecological Indicators, 2014, 45: 209-218. DOI:10.1016/j.ecolind.2014.04.008 (  0) 0) |
| [24] |
He W, Qin N, Kong X Z, et al. Water quality benchmarking (WQB) and priority control screening (PCS) of persistent toxic substances (PTSs) in China: Necessity, method and a case study[J]. Science of the Total Environment, 2014, 472: 1108-1120. DOI:10.1016/j.scitotenv.2013.11.119 (  0) 0) |
| [25] |
Solomon K, Giesy J, Jones P. Probabilistic risk assessment of agrochemicals in the environment[J]. Crop Protection, 2000, 19(8-10): 649-655. DOI:10.1016/S0261-2194(00)00086-7 (  0) 0) |
| [26] |
DeLorenzo M E, Leatherbury M, Weiner J A, et al. Physiological factors contributing to the species-specific sensitivity of four estuarine microalgal species exposed to the herbicide atrazine[J]. Aquatic Ecosystem Health and Management, 2004, 7(1): 137-146. DOI:10.1080/14634980490281551 (  0) 0) |
| [27] |
Debelius B, Forja J M, Del V A, et al. Effect of linear alkylbenzene sulfonate (LAS) and atrazine on marine microalgae[J]. Marine Pollution Bulletin, 2008, 57(6-12): 559-568. DOI:10.1016/j.marpolbul.2008.01.040 (  0) 0) |
| [28] |
Magnusson M, Heimann K, Negri A P. Comparative effects of herbicides on photosynthesis and growth of tropical estuarine microalgae[J]. Marine Pollution Bulletin, 2008, 56(9): 1545-1552. DOI:10.1016/j.marpolbul.2008.05.023 (  0) 0) |
| [29] |
DeLorenzo M E, Serrano L. Individual and mixture toxicity of three pesticides; atrazine, chlorpyrifos, and chlorothalonil to the marine phytoplankton species Dunaliella tertiolecta[J]. Journal of Environmental Science and Health, 2003, 38(5): 529-538. DOI:10.1081/PFC-120023511 (  0) 0) |
| [30] |
Gaggi C, Sbrilli G, Naby A, et al. Toxicity and hazard ranking of s-triazine herbicides using microtox, two green algal species and a marine crustacean[J]. Environmental Toxicology and Chemistry, 1995, 14(6): 1065-1069. (  0) 0) |
| [31] |
Doherty M A. Biochemical Toxicology of Herbicide Mixtures on Thalassiosira weisflogii[D]. Washington: University of Maryland, College Park, 1997.
(  0) 0) |
| [32] |
Walsh G E, McLaughlin L L, Yoder M J, et al. Minutocellus polymorphus: A new marine diatom for use in algal toxicity tests[J]. Environmental Toxicology and Chemistry, 1988, 7(11): 925-929. (  0) 0) |
| [33] |
Hoberg J R. Toxicity to the Marine Diatom, Skeletonema costatum[R]. Wareham: Springborn Laboratories Incorpration, 1998.
(  0) 0) |
| [34] |
Wang Z, Sun X, Ru S, et al. Effects of co-exposure of the triazine herbicides atrazine, prometryn and terbutryn on Phaeodactylum tricornutum photosynthesis and nutritional value[J]. Science of the Total Environment, 2022, 807: 150609. DOI:10.1016/j.scitotenv.2021.150609 (  0) 0) |
| [35] |
González-Barreiro O, Rioboo C, Cid A, et al. Atrazine-induced chlorosis in Synechococcus elongatus cells[J]. Archives of Environmental Contamination and Toxicology, 2004, 46(3): 301-307. (  0) 0) |
| [36] |
Pennington P L, Scott G I. Toxicity of atrazine to the estuarine phytoplankter Pavlova sp. (Prymnesiophyceae): Increased sensitivity after long-term, low-level population exposure[J]. Environmental Toxicology and Chemistry, 2001, 20(10): 2237-2242. (  0) 0) |
| [37] |
Crisinel A, Delaunay L, Rossel D, et al. Cyst-based ecotoxicological tests using anostracans: Comparison of two species of streptocephalus[J]. Environmental Toxicology and Water Quality, 1994, 9(4): 317-326. DOI:10.1002/tox.2530090411 (  0) 0) |
| [38] |
Wilkins R M, and Metcalfe R J. Toxicity of soil applied herbicides to brine shrimp larvae (Artemia salina) and synergism with other pesticides[C]//British Crop Protection Conference. Thornton Heath: British Crop Protection Council, 1993: 163-168.
(  0) 0) |
| [39] |
Ward G S, Ballantine L. Acute and chronic toxicity of atrazine to estuarine fauna[J]. Estuaries, 1985, 8(1): 22-27. DOI:10.2307/1352118 (  0) 0) |
| [40] |
United States Environmental Protcction Agency. Pesticide Ecotoxicity Database (Formerly: Environmental Effects Database (EEDB))[R]. Washington: Environmental Fate and Effects Division, 1992.
(  0) 0) |
| [41] |
Hack L A, Tremblay L A, Wratten S D, et al. Zinc sulfate and strazine toxicity to the marine harpacticoid copepod Robertsonia propinqua[J]. New Zealand Journal of Marine and Freshwater Research, 2008, 42(1): 93-98. DOI:10.1080/00288330809509939 (  0) 0) |
| [42] |
Stringer T J, Glover C N, Keesing V, et al. Development of a harpacticoid copepod bioassay: Selection of species and relative sensitivity to zinc, atrazine and phenanthrene[J]. Ecotoxicology and Environmental Safety, 2012, 80: 363-371. DOI:10.1016/j.ecoenv.2012.04.008 (  0) 0) |
| [43] |
Phyu Y L, Warne M S, Lim R P. Toxicity and bioavailability of atrazine and molinate to the freshwater shrimp (Paratya australiensis) under laboratory and simulated field conditions[J]. Ecotoxicology and Environmental Safety, 2005, 60(2): 113-122. DOI:10.1016/j.ecoenv.2004.07.006 (  0) 0) |
| [44] |
Noppe H, Ghekiere A, Verslycke T, et al. Distribution and ecotoxicity of chlorotriazines in the Scheldt Estuary (B-N l)[J]. Environmental Pollution, 2007, 147(3): 668-676. DOI:10.1016/j.envpol.2006.09.016 (  0) 0) |
| [45] |
Forget J, Pavillon J F, Menasria M R, et al. Mortality and LC50 values for several stages of the marine copepod Tigriopus brevicornis (Müller) exposed to the metals arsenic and cadmium and the pesticides atrazine, carbofuran, dichlorvos, and malathion[J]. Ecotoxicology and Environmental Safety, 1998, 40(3): 239-244. DOI:10.1006/eesa.1998.1686 (  0) 0) |
| [46] |
McNamara P C. Atrazine-Acute Toxicity to the Marine Copepod (Acartia tonsa) Under Flow-Through Conditions[R]. Greensboro, NC: Ciba-Geigy Corporation, 1991.
(  0) 0) |
| [47] |
Key P, Chung K, Siewicki T, et al. Toxicity of three pesticides individually and in mixture to larval grass shrimp (Palaemonetes pugio)[J]. Ecotoxicology and Environmental Safety, 2007, 68(2): 272-277. DOI:10.1016/j.ecoenv.2006.11.017 (  0) 0) |
| [48] |
Bejarano A C, Chandler G T. Reproductive and developmental effects of atrazine on the estuarine meiobenthic copepod Amphiascus tenuiremis[J]. Environmental Toxicology and Chemistry, 2003, 22(12): 3009-3016. DOI:10.1897/03-40 (  0) 0) |
| [49] |
Machado M W. Atrazine Technical-Acute Toxicity to Mysid Shrimp (Mysidopsi sbahia) Under Flow-Through Conditions[R]. Wareham: Springborn Laboratories Incorporation, 1994.
(  0) 0) |
| [50] |
Portmann J E, Wilson K W. The Toxicity of 140 Substances to the Brown Shrimp and Other Marine Animals[R]. North Wales: Burnham-on-Crouch, 1971.
(  0) 0) |
| [51] |
Mhadhbi L, Hela T, Moncef B, et al. Toxicity of three selected pesticides (Alachlor, Atrazine and Diuron) to the marine fish (Turbot Psetta maxima)[J]. African Journal of Biotechnology, 2012, 11(51): 11321-11328. (  0) 0) |
| [52] |
Hall L W, Ziegenfuss M C, Anderson R D, et al. Influence of salinity on atrazine toxicity to a Chesapeake Bay copepod (Eurytemora affinis) and fish (Cyprinodon variegatus)[J]. Estuaries, 1994, 17(1B): 181-186. (  0) 0) |
| [53] |
Wilkinson A D, Collier C J, Flores F, et al. Acute and additive toxicity of ten photosystem-Ⅱ herbicides to seagrass[J]. Scientific Reports, 2015, 5: 17443. DOI:10.1038/srep17443 (  0) 0) |
| [54] |
Lawton J C, Pennington P L, Chung K W, et al. Toxicity of atrazine to the juvenile hard clam, Mercenaria mercenaria[J]. Ecotoxicology and Environmental Safety, 2006, 65(3): 388-394. DOI:10.1016/j.ecoenv.2005.08.001 (  0) 0) |
| [55] |
Pennington P L. The Replicated Modular Estuarine Mesocosm: Assessing Direct and Indirect Effects of Pesticide Exposure[D]. Columbia: University of South Carolina, 2002.
(  0) 0) |
| [56] |
Hall L W, Ziegenfuss M C, Anderson R D, et al. The influence of salinity on the chronic toxicity of atrazine to an estuarine copepod: Implications for development of an estuarine chronic criterion[J]. Archives of Environmental Contamination and Toxicology, 1995, 28(3): 344-348. DOI:10.1007/BF00213112 (  0) 0) |
| [57] |
Le Mer C, Roy R L, Pellerin J, et al. Effects of chronic exposures to the herbicides atrazine and glyphosate to larvae of the three spine stickleback (Gasterosteus aculeatus)[J]. Ecotoxicology and Environmental Safety, 2013, 89: 174-181. DOI:10.1016/j.ecoenv.2012.11.027 (  0) 0) |
| [58] |
Hall L W, Anderson Jr. R D, Ailstock M S. Chronic toxicity of atrazine to sago pondweed at a range of salinities: Implications for criteria development and ecological risk[J]. Archives of Environmental Contamination and Toxicology, 1997, 33(3): 261-267. DOI:10.1007/s002449900252 (  0) 0) |
| [59] |
Scott C H. A Comparison of Vegetation Indices and Conventional Ecotoxicological Plant Growth Metrics to Assess the Lethal and Sublethal Effects of Atrazine in Smooth Cordgrass, Spartina alterniflora[D]. Columbia: University of South Carolina, 2011.
(  0) 0) |
| [60] |
Sawasdee B, Köhler H R. Embryo toxicity of pesticides and heavy metals to the ramshorn snail, Marisa cornuarietis (Prosobranchia)[J]. Chemosphere, 2009, 76(7): 1016. DOI:10.1016/j.chemosphere.2009.06.001 (  0) 0) |
| [61] |
Lawton J C, Pennington P L, Chung K W, et al. Toxicity of atrazine to the juvenile hard clam, Mercenaria mercenaria[J]. Ecotoxicology and Environmental Safety, 2006, 65(3): 388-394. DOI:10.1016/j.ecoenv.2005.08.001 (  0) 0) |
| [62] |
El-shenawy N S, Greenwood R, Abdel-Nabi I M. Histological response of purple mussels to long-term exposure to sublethal doses of lindane and atrazine[J]. Journal of Animal, 2007(5): 899-909. (  0) 0) |
| [63] |
郑磊, 杨文龙, 董亮, 等. 扑草净水环境质量基准及风险评估[J]. 中国环境科学, 2021, 41(8): 3825-3831. Zheng L, Yang W L, Dong L, et al. Water quality criteria and risk assessment of prometryn in the fresh water[J]. China Environmental Sciences, 2021, 41(8): 3825-3831. DOI:10.3969/j.issn.1000-6923.2021.08.040 (  0) 0) |
| [64] |
Sun C, Xu Y, Hu N, et al. To evaluate the toxicity of atrazine on the freshwater microalgae Chlorella sp. using sensitive indices indicated by photosynthetic parameters[J]. Chemosphere, 2020, 244: 125514. DOI:10.1016/j.chemosphere.2019.125514 (  0) 0) |
| [65] |
秦璐, 宋秀凯, 刘丽娟, 等. 高效氟吡甲禾灵对海洋生物的急性毒性与水质基准推导[J]. 环境科学研究, 2022, 35(6): 1509-1518. Qin L, Song X K, Liu L J, et al. Acute toxicity and water quality criteria of high potency flurpyrimethalin to marine organisms[J]. Environmental Science Research, 2022, 35(6): 1509-1518. (  0) 0) |
| [66] |
Moore D R, Priest C D, Galic N, et al. Correcting for phylogenetic autocorrelation in species sensitivity distributions[J]. Integrated Environmental Assessment and Management, 2020, 16(1): 53-65. DOI:10.1002/ieam.4207 (  0) 0) |
| [67] |
高亚平, 蒋增杰, 杜美荣, 等. 除草剂扑草净和阿特拉津对海草与大型藻类的毒性比较[J]. 水生生物学报, 2017, 41(4): 930-934. Gao Y P, Jiang Z J, Du M R, et al. Comparison of toxicity of the herbicides prometryn and atrazine to seagrass and macroalgae[J]. Journal of Hydrobiology, 2017, 41(4): 930-934. (  0) 0) |
| [68] |
Jiang L, Sullivan H, Seligman C, et al. An NMR-based metabolomics study on sea anemones Exaiptasia diaphana (Rapp, 1829) with atrazine exposure[J]. Molecular Omics, 2021, 17(6): 1012-1020. DOI:10.1039/D1MO00223F (  0) 0) |
| [69] |
徐英江, 刘慧慧, 任传博, 等. 莱州湾海域表层海水中三嗪类除草剂的分布特征[J]. 渔业科学进展, 2014, 35(3): 34-39. Xu Y J, Liu H H, Ren C B, et al. Distribution characteristics of triazine herbicides in surface waters of Laizhou Bay[J]. Advances in Fishery Science, 2014, 35(3): 34-39. (  0) 0) |
| [70] |
Zhang R, Du J, Dong X, et al. Occurrence and ecological risks of 156 pharmaceuticals and 296 pesticides in seawater from mariculture areas of Northeast China[J]. Science of the Total Environment, 2021, 792: 148375-148375. DOI:10.1016/j.scitotenv.2021.148375 (  0) 0) |
2. Shandong Provincial Key Laboratory of Restoration for Marine Ecology, Shandong Marine Resources and Environment Research Institute, Yantai 264006, China
 2024, Vol. 54
2024, Vol. 54


