2. 青岛海洋科学与技术试点国家实验室海洋渔业科学与食物产出过程功能实验室, 山东 青岛 266237
性别是最普遍的生物学现象,许多生物的雌性和雄性在形态、生殖策略和行为上存在显著差别。性别的形成受到性别决定的控制,又受到性腺分化的影响。性别决定与性腺分化相互联系又有所区别;性别决定是确定性腺分化趋向,而性腺分化是具双向潜力的未分化性腺经过程序性发生的一系列事件,发育成精巢或卵巢,并出现第二性征的过程。
硬骨鱼作为低等的脊椎动物,是脊椎动物中最大类群,其性别决定机制和性别分化呈现出多样性,包括遗传决定(GSD)、环境因素决定(ESD)和两者共同决定(GSD + ESD)等模式[1-3]。此外,硬骨鱼的性别表型不但有雌雄异体,还存在雌雄同体、性逆转等特殊的生物学现象。
硬骨鱼独有的以上特点,为研究生物的性别决定和性腺分化等生物学过程提供了丰富的遗传材料,可作为理想的模式生物来研究性别相关的生物学过程。阐明硬骨鱼的性别决定方式,不仅有助于完善生物性别决定的基础知识,对基础研究贡献巨大,在生产上通过性别控制育种也能达到增产效果,具有重要应用价值。鉴于鱼类性别决定研究在理论知识和生产实践中均具有重要意义,国内外科研人员在一系列硬骨鱼中展开相关研究,从模式物种斑马鱼(Danio rerio)、青鳉(Oryzias latipes),到大型经济物种尼罗罗非鱼(Oreochromis niloticus)、大西洋鲑(Salmo salar)、半滑舌鳎(Cynoglossus semilaevis)和河鲀(Takifugu rubripes)等均取得了突破性进展[4-9]。
半滑舌鳎属于鲽形目(Pleuronectiformes)舌鳎科(Cynoglossidae)舌鳎属(Cynoglossus)是中国黄渤海地区近海底栖鱼类,也是重要的经济物种。该物种雌雄个体差异巨大,雌性成体个体体长是雄性个体的2~4倍,雌性成熟个体的体重可达雄性的8~10倍[10-11]。目前半滑舌鳎工厂化养殖在中国北方地区发展迅速,已成为规模化养殖的优势品种之一。然而,由于半滑舌鳎繁殖周期长,且雌雄个体规格差异巨大,在同等条件下,如果养殖全雌鱼或高雌性比例群体,可极大提高单位面积产量,进而获得更高的经济效益。因此,研究半滑舌鳎性别决定的分子机制,为开展人工性别控制、单性品种或高雌性比例的养殖群体等提供理论基础。
已有研究表明,半滑舌鳎有明显的性染色体,属于ZZ/ZW性染色体类型[12],同时其性别决定不仅受遗传因素决定,还受到环境因素的调控[8]。另外研究人员通过筛选半滑舌鳎雌性特异分子标记,可以准确的识别出其遗传性别[13-14]。该物种独特的生物学特征为研究硬骨鱼的性别决定提供了理想的模型。本综述就半滑舌鳎性别决定、环境因素诱导的性逆转现象、性别控制育种等方面取得的重要进展和研究成果分别进行综合介绍,并与其他鱼类在相关领域的研究进展进行比较分析。
1 半滑舌鳎性别决定研究进展性别决定由性别决定基因启动,当性别决定基因表达后,经过一系列级联反应,使生物体表现出雌雄性别表型。目前为止,仅在少量物种中鉴别出性别决定基因。在高等脊椎动物的性别决定基因比较单一,但在硬骨鱼中,性别决定系统异常复杂,其性别决定基因呈现出多样性,到目前为止在硬骨鱼中发现的性别决定基因如表 1所示。
|
|
表 1 硬骨鱼性别决定基因一览表 Table 1 Reported sex differentiation genes in teleost |
对半滑舌鳎性别决定的研究起始于该物种存在性别二态性,雌性个体远大于雄性。周丽青等首先通过染色体观察证明,半滑舌鳎有明显的性染色体,其性别决定属于ZZ/ZW类型,雌性个体拥有一个异形的W染色体[12]。在近二十年的研究中,通过差减文库分析、基因克隆和性腺发育各时期表达量的变化等技术手段,发现和解析了一系列在雌雄性腺中表现出差异表达的基因,包括Amh、Amhr2、Wnt1a/b、Dmrt1、Gsdf、Sox9a、Dazl、Sf1、Figla、Sox10、Foxl2、Zp3a/b、cyp19a1a、GATA、Rspodin1等[8, 10, 20-27]。这些呈现出雌雄二态性表达的基因,部分偏向于雄性或雌性表达,部分表现为在整个生殖周期过程中动态表达,其功能涉及类固醇激素的合成和调控、性腺发育、配子发生、成熟过程的调节等。
伴随高通量测序技术的普及,半滑舌鳎转录组、基因组和甲基化组测序等技术平台都相继成熟,为后续性别决定基因的筛选提供了更为丰富的参考信息。通过对半滑舌鳎精巢和卵巢等转录组的测序分析,筛选出大量雌雄表达差异的基因[28]。随着半滑舌鳎基因组测序完成,结合雌鱼、雄鱼和伪雄鱼转录组和甲基化组分析,半滑舌鳎的性别决定基因筛选范围进一步缩小,初步认为Dmrt1基因是半滑舌鳎的性别决定基因[8, 28-29]。Cui等用基因编辑技术对半滑舌鳎Dmrt1基因进行了基因编辑,发现ZZ型突变体的性腺发育成卵巢样精巢,且精子发生过程被阻断,该突变体比野生型ZZ个体表现出更快的生长速度,其形体和大小与野生型ZW雌鱼类似。在基因编辑的突变体中,研究人员还发现ZW型雌雄同体突变体,上下两侧性腺分别呈现出精巢和卵巢结构。分子生物学检测发现,上侧精巢中未发现Dmrt1突变,而下侧卵巢的性腺中56%的Dmrt1基因发生了突变,推断这是一个基因编辑的伪雄鱼嵌合体。通过上述基因编辑结果,Cui等认为Dmrt1基因是半滑舌鳎雄性决定基因,同时也是雄性精巢发育必不可少的[18]。
Dmrt1基因属于Dmrt基因家族,包含典型性的DM结构域,一系列研究表明该基因在哺乳类、鸟类、爬行类、两栖类和鱼类中与性别决定与性腺分化、雄性性腺的发育和维持密切相关[30-35]。在许多物种中,Dmrt1基因已被广泛研究,包括牙鲆(Paralichthys olivaceus)、尼罗罗非鱼、虹鳟(Oncorhynchus mykiss)、黄颡鱼(Pelteobagrus fulvidraco)、点带石斑鱼(Epinephelus coioides)、青鳉、稀有鮈鲫(Gobiocypris rarus)、东方红鳍鲀、革胡子鲶(Clarias gariepinus)、黑鲷(Acanthopagrus schlegeli)、许氏平鮋(Sebastes schlegelii)和斑马鱼等,该基因在大部分硬骨鱼中只在精巢和卵巢中表达,且在精巢中的表达量显著高于精巢[31, 34, 36-45]。在以前的研究中发现,青鳉Dmrt1的同源基因DMY是雄性性别决定基因,该基因是Dmrt1基因发生复制后转移Y染色体上产生的,当DMY基因突变或过表达后,都会出现性逆转现象[6-7]。与青鳉类似,Yoshimoto等在对两栖动物光滑爪蟾(Xenopus leavi)的研究中也发现,DMW基因是其性别决定基因,该基因是Dmrt1在W染色体上的一个特殊拷贝,当DMW基因过表达或敲降后,会表现出性逆转现象[46-47]。
在半滑舌鳎中,Dmrt1基因已被克隆,该基因由5个外显子和4个内含子组成。组织表达模式分析发现,Dmrt1基因是雄性精巢特异表达基因。在不同发育时期,Dmrt1基因在雌雄个体中呈现出显著差别,在雌鱼性腺中基因检测不到Dmrt1基因的表达。在雄鱼中,从发育到48天的仔鱼中检测到Dmrt1开始表达,70天表达开始升高,1年龄时,达到最高值[48-49]。染色体定位和基因组分析发现,该基因位于Z染色体上,原位杂交结果显示,Dmrt1主要在精巢的生殖细胞(Germ cell)和将来分化成支持细胞的体细胞(Presomatic cell)中表达[29]。这些结果都表明Dmrt1在半滑舌鳎性别决定或(和)性腺分化中发挥了重要作用。但是,单纯根据少数基因编辑个体的表型就断定Dmrt1是其性别决定基因这一结论有待进一步确认。首先,基因编辑的突变体都是原代个体,个体数量不明;再者,基因编辑发生在受精卵或胚胎发育的早期,而性腺分化是在变态后的幼鱼时期,基因编辑如何能够获得上述一侧性腺被编辑雌雄同体个体,或者说基因编辑的细胞为什么只在一侧性腺中出现,尚无法解释;此外,当Dmrt1基因过表达后,会出现怎样的性别表型变化,亦有待进一步研究。
2 环境因素诱导的性逆转现象在高等脊椎动物中,性别一旦形成,一般比较稳定,不容易发生性逆转现象。在低等脊椎动物中,其性别由遗传物质和环境因子共同决定,鱼类作为低等的脊椎动物,在发育过程中其性别的可塑性较大,除了遗传因素的调控外,外界环境对其性别分化的影响也非常大。外界环境因子中的温度对性别分化的影响尤其重要。在鱼类中,性腺分化过程中由于环境因素影响而分化为假雄鱼和假雌鱼这一现象被称为性逆转现象(Sex reversal),产生的假雄鱼或者假雌鱼被成为伪雄鱼或者伪雌鱼。大西洋银汉鱼(Menidia menidia)是首先被发现温度能改变性别比例的物种[50],随后的研究中发现很多硬骨鱼中发现类似现象[1, 3]。性别比例的偏离主要是性逆转造成的,一般情况下是雌鱼性逆转成伪雄鱼,导致雄性比例偏高。半滑舌鳎性逆转现象产生的原因也多是环境温度的变化过高或过低温度能诱导雄鱼的产生[1-2, 51]。本实验室对不同养殖场来源的生理雄鱼进行了遗传鉴定,并跟踪调查了这些养殖群体苗种生产企业苗种培育水温情况,结果发现,在保苗阶段,或者说在苗种性别分化阶段,不同的苗种培育温度对后代的生理性别产生较大影响(见表 2)。温度越高,伪雄鱼的比例也越高,育苗水温达到24 ℃时,生理雄鱼中伪雄鱼的比例可达37%。保苗温度与伪雄鱼比例间存在极显著的正相关关系(P=0.986)。
|
|
表 2 半滑舌鳎不同人工养殖群体的生理雄鱼中伪雄鱼的比例 Table 2 Detection of pseudomales in different cultured stocks of Chinese tongue sole |
一系列研究发现,高温或雄性激素诱导以及养殖群体中自然性逆转的伪雄鱼,其表现出的精巢性状跟雄鱼类似[8, 48, 52-53],在进行相关遗传学研究时必须对其进行区分。由于半滑舌鳎存在明显的W性染色体,且其较大易于区分,Wang等通过染色体切割技术对W染色体进行了切割,建立了W染色体特异的基因组文库,从而开发了雌性特异的分子标记[54];另有研究分别开发了性别特异的AFLP标记和SSR标记[55-56]。这些性别特异分子标记的开发为鉴别半滑舌鳎的遗传性别提供了有利工具,结合表型性别的观察,可以准确的鉴定雌鱼、雄鱼和伪雄鱼(见图 1)。
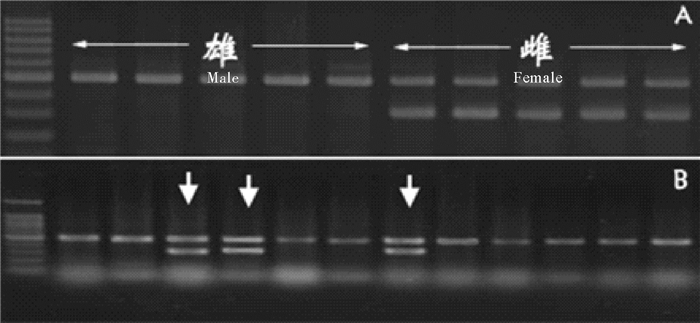
|
( A.遗传雌雄的PCR扩增结果,雌性有特异的条带;B.养殖群体的生理雄鱼中伪雄鱼的识别,箭头所指为伪雄鱼。A.PCR amplification results in genetic males and females, females with a specific band. B.Identification of pseudomales from physiological males. Arrows show the pseudomales with a female specific band.) 图 1 半滑舌鳎遗传性别的分子鉴定 Fig. 1 Genetic sexing of Chinese tongue sole |
性逆转产生的伪雄鱼在遗传性别上和雌鱼相同,都是ZW型个体,但表型和ZZ型雄性个体相似。深入的分析发现,性逆转个体的表观遗传特性发生了巨大的变化。全基因组甲基化测序发现伪雄鱼和雄鱼基因组甲基化水平整体比较相似,都表现出较高的甲基化水平,与正常雌鱼相比甲基化水平差异显著[8]。半滑舌鳎性逆转个体不仅在基因组整体上甲基化水平具有显著差异,对性别相关基因包括piwi、GATA4、GATA6、cyp19a1a等的启动子区甲基化水平进行分析发现,在不同性别的个体中这些基因的启动子区甲基化水平不同,其甲基化模式也存在显著差别,同时发现这些基因的表达量的变化和甲基化水平呈现出相关性[23-24, 52, 57]。Liu等最近的研究发现,半滑舌鳎伪雄鱼cyp19a1a启动子区的甲基化水平是雌鱼中的两倍,而且和雄鱼的甲基化模式类似。体外实验结果表明,cyp19a1a启动子区甲基化水平可影响转录因子CREB的结合并调控该基因的转录,进一步影响雌雄类固醇激素的比例,从而引起下游一系列级联反应,进而影响性别表型[52]。
本实验室前期研究表明,伪雄鱼能够产生有功能的配子,但受精率和孵化率显著低于正常雄鱼来源的精子,其后代的生长性状与正常雄鱼后代存在显著差异[58]。Shao等研究发现伪雄鱼产生的后代生长速率缓慢,同时证明了伪雄鱼的表观遗传改变可通过遗传物质传递给子代个体[8]。在正常的养殖群体中,半滑舌鳎只有部分雌鱼会发生性逆转发育伪雄鱼,Jiang和Li通过GWAS分析发现,位于Z染色体上的FBXL17基因的SNP位点(A/T)与该现象相关,ZAW型雌鱼不会发生性逆转现象,但ZTW型雌鱼较为敏感,在外界环境的影响下容易发生性逆转[59]。在半滑舌鳎Dmrt1基因第三内含子的SNP(A/G)也有类似现象,当该SNP的基因型为T时,雌性个体易发生性逆转[60]。综合上述研究结果,可以推测伪雄鱼的产生受到表观遗传修饰的调控,某些基因的SNP突变,会引起对环境敏感程度的差别,带有敏感突变的个体更容易受到环境因子的影响而发生性逆转。环境因素诱导的性逆转,尤其是温度对性别的影响,在两栖类和爬虫动物中也有发现[61-62]。关于性逆转现象产生的分子机制被广泛研究,不同学者从不同的角度对该问题进行了深入探讨,发现类固醇激素、糖皮质激素和表观遗传修饰等因素在性逆转过程中发挥至关重要的作用[63-71]。在爬行类中,Deveson等发现在JARID2和JMJD3剪切过程中内含子保留(Intron retention,IR)可介导澳大利亚蜥蜴(Pogona vitticeps)的性逆转现象[72]。在温度敏感型巴西红耳龟(Trachemys scripta)和美洲鳄(Alligator mississippiensis)中,cyp19a1a能调控类固醇激素的比例,从而改变表型性别[73-74]。在硬骨鱼中,关于高温诱导后趋向雄性化这一现象和表观遗传修饰之间的关系在欧洲鲈(Dicentrarchus labrax)、尼罗罗非鱼、虹鳟和半滑舌鳎中已陆续展开,研究发现cyp19a1a基因启动子区甲基化水平与性别密切联系[52, 69, 75-76]。在牙鲆中发现FOXL2和SOX9甲基化也呈现出性别二态性[77]。另外,HSPs、TRPs、CIRBPs和microRNA在环境因子对性别的影响过程中发挥重要作用[78-82]。在雌雄同体的尖吻鲈(Lates calcarifer)中dmrt1和cyp19a1不仅存在性别特异的甲基化水平,还存性别特异的剪切[83]。
上述的研究表明,环境因子与遗传信息互作对半滑舌鳎等许多硬骨鱼性别分化的影响体现在表观遗传修饰上,通过表观遗传修饰,可调控性别决定或性腺分化相关基因及信号通路的表达,从而影响性腺分化和配子发生。然而,在环境影响基因表达调控方面,非编码RNA的功能却少有涉及。已有的研究表明,在斑石鲷(Oplegnathus punctatus)miR-27b-3p直接靶向piwi2和mov10l1的3’UTR并下调其表达[84];miR-141和miR-429在黄颡鱼精巢发育和精子发生中发挥重要作用[85];在黄鳝(Monopterus albus)中,miR-19a/b能够直接抑制性逆转期dmrt1的表达[86];牙鲆piRNA信号通路基因经历了快速的进化,它们在雄性精巢中高表达,与雄性偏向的piwi基因的高表达相协调,可能在精巢发育和精子发生中发挥重要功能[87]。这些非编码RNA是否直接受到环境因子变化的影响,以及相关变化怎样通过影响相关靶基因的表达调控性腺分化等,均未见报道。因此,在以后的研究中转录水平上非编码RNA对性别分化的调控是值得探讨的内容。此外,性别分化相关基因在蛋白水平上磷酸化、乙酰化修饰等对性别分化的影响也有待探讨。
3 半滑舌鳎性别控制育种半滑舌鳎雌性个体远大于雄性个体,在养殖过程具较高的经济价值,提高养殖群体的雌性比例或培育全雌苗种对半滑舌鳎的养殖产业意义巨大。对于ZZ/ZW性别决定型的半滑舌鳎来说,培育全雌苗种的理想方法是,首先获得WW染色体组成的超雌鱼,然后通过WW超雌鱼与普通ZZ雄鱼交配,获得ZW全雌后代。获得WW超雌鱼的有效途径有两个,一是开展雌核发育,能够获得ZZ和WW两种雌核发育个体;另一个是培育或筛选ZW伪雄鱼,通过伪雄鱼与正常ZW雌鱼交配,理论上可以获得的后代中有四分之一为WW超雌鱼。
3.1 半滑舌鳎雌核发育研究本实验室通过采集ZZ型雄鱼的精子,并参考戈文龙等的方法用紫外线进行失活处理,利用静水压力方法诱导雌核发育[88],成功获得了多批次雌核发育个体。待雌核发育个体生长至5 cm左右时,随机选取94尾,提取DNA样本,与雌性亲本及提供精子的雄性亲本一起进行PCR分子遗传性别鉴定。结果表明,所有雌核发育后代的遗传性别均与雄性亲本一致,而与雌性亲本不同,缺少W特异的分子标记(见图 2A ),随机选取10尾进行染色体观察显示,所有个体的染色体组成均为ZZ型(见图 2 C),没有观察到雌雄个体应有的W染色体(见图 2B )。这一结果表明,雌核发育后代全部为ZZ型遗传雄性,WW型雌性个体不能存活。Chen等报道在其雌核发育研究时,孵化2天的仔鱼中发现有WW超雌鱼苗[55],但未对较大的幼鱼进行检测。显然,WW超雌鱼未能成活到5 cm左右的稚鱼,那么,WW雌鱼到底什么时候死亡的,有待进一步探讨。
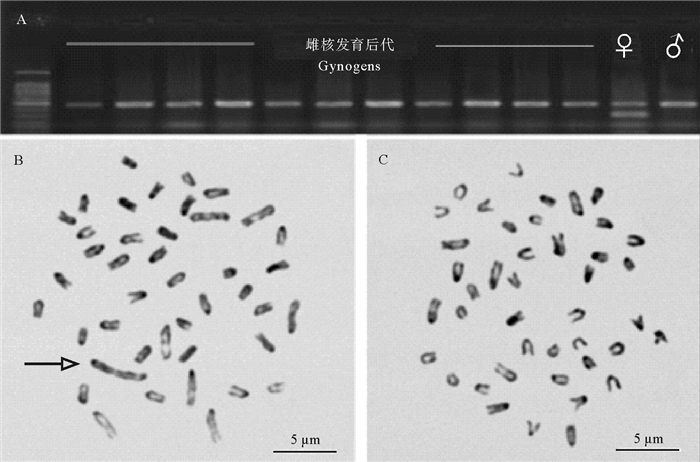
|
(A:雌核发育用雌性亲本、雄性亲本及获得后代的分子标记检测,所有后代全部为雄性;B:一般雌雄个体的染色体,箭头示W染色体;C:雌核发育后代的染色体,全部为ZZ型。A: PCR amplification of female and male parents and the gynogens, all gynogens do not have the female specific marker. B: Chromosomes of normal female individual with arrow indicating the W chromosome. C: Chromosomes of diploid gynogen, with all individuals show ZZ chromosomes.) 图 2 半滑舌鳎雌核发育后代的性别特异分子标记检测及染色体观察 Fig. 2 Identification of gynogens with female specific marker and chromosome observation |
利用雌性特异标记筛选方法[14, 54],于2006—2007年分别从2005和2006年培育的苗种中筛选获得伪雄鱼817尾(见表 2),分别于2007和2008年进行了伪雄鱼后代的培育,共培育苗种5万余尾。至苗种一月龄时,分别从2007和2008年度的苗种中各取400尾,用雌雄特异分子标记后代进行了遗传性别鉴定,结果显示,2007年度伪雄鱼的后代中,遗传雌性的比例为53.6%,同期普通对照组雌性比例为47%,两者没有显著差异;2008年度伪雄鱼的后代中,遗传雌性的比例为48.5%,同期普通对照组雌性比例为48.3%,两者也没有显著差异[58]。
从分子标记确认的一月龄遗传雌性仔鱼中,随机挑取200尾进行了染色体观察,结果有167尾成功地观察点清晰可辨核型的染色体,这些个体全部为ZW型遗传雌性,未观察到WW个体的存在。在前期利用FISH杂交研究rDNA分布时发现,在半滑舌鳎雄性染色体组中有3对杂交信号,而在雌雄染色体组中,有7个明显的杂交信号,除与雄性相同的3对信号外,在W上有一个较大区段的rDNA分布(见图 3)。为了验证对伪雄鱼后代分子鉴定及染色体观察的结果,以18S rDNA为探针,对观察到的染色体进行原位杂交,结果发现,所有观察到的遗传雌性个体的染色体组中,都有且只有7个杂交信号,包括3对成对的信号和一个W染色体特异信号(见图 3)。充分证明,在伪雄鱼的后代中,不存在WW超雌鱼。
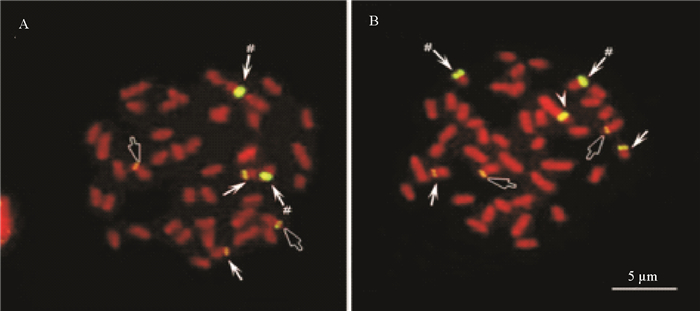
|
(A:在雄性染色体组中有3对杂交信号,分别为箭头、#箭头和空箭头;B:雌性染色体组中有7个杂交信号,小箭头示W染色体特异rDNA信号。伪雄鱼的所有雌性后代染色体组都与B相同。A: Male karyotype shows 3 pairs of rDNA bearing chromosomes, arrows, # arrows and hollow arrows; B: Female karyotype shows 3 pairs of rDNA bearing chromosomes and an additional big W chromosome signal.) 图 3 18S rDNA在半滑舌鳎染色体组中的分布 Fig. 3 Distribution of 18S rDNA in the chromosomes of Chinese tongue sole |
对2007年度培育的苗种进行了养殖测试,2008年度培育的苗种,因与2007年度组一样,经过遗传鉴定也没有发现WW超雌鱼,所以,直接放弃养殖测试。结果还发现,伪雄鱼后代生长速度显著低于普通组,至7月龄时,伪雄鱼后代中遗传雌性个体的平均体长为18.3 cm,对照组为22.2 cm,对照组比伪雄组大21.3%,伪雄组和对照组遗传雄性个体的平均体长分别为16.7和17.6 cm,两者没有明显差异(见表 3),因此,伪雄鱼后代在整体是生长较慢是由于其中遗传雌雄个体生长速度较慢造成的。
|
|
表 3 半滑舌鳎伪雄鱼后代中遗传雌雄个体的比例及其生长状况比较 Table 3 The percentage and the growth rate of male and female offspring of pseudomales |
首先,关于伪雄鱼后代的雌雄比例,本研究发现其雌雄比为1:1,与普通雄鱼后代比例一致。理论上说,ZW×ZW杂交后代的比例为ZZ:ZW:WW=1:2:1,由于后代中不存在WW个体,且ZW个体与ZZ个数相等,因此,很容易想到杂交亲本中一方来源的W配子不存在或不能正常受精发育。事实上,Cui等后来的研究证实,伪雄鱼不能产生W型精子[60],这证实了本研究的观察结果,即:WW超雌个体是不存在的,通过伪雄鱼培育WW超雌鱼进而培育全雌品种的途径是行不通的。
关于伪雄鱼后代的的生长速度,发现伪雄鱼后代生长速度明显较对照组慢,且这些差异主要是由于伪雄鱼后代中遗传雌性的生长速度慢造成的。Shao等研究也发现伪雄鱼的后代生长缓慢,且其中生理雄性比例极高[8],后来,Jiang和Li证实,在半滑舌鳎Z染色体上的FBXL17基因存在一个SNP位点(A/T),ZTW型个体对外界温度变化较为敏感,更容易发生性逆转[59],Dmrt1基因第三内含子的SNP(A/G)也与雌性个体温度易感性相关[60]。因此,伪雄鱼这一性状是遗传的,容易发生性逆转变成伪雄鱼的个体,多数携有温度敏感型SNP位点,其后代也继承了相应SNP位点,也容易发生性逆转成为伪雄鱼。
3.3 半滑舌鳎三倍体的培育与养殖实验由于半滑舌鳎性染色体结构的特殊性,使得全雌苗种的诸多尝试均未获得理论上预料的成果,因为许多动物中都已证实三倍体比二倍体有一定的生长优势[89-91],国内不少学尝试了半滑舌鳎多倍体育种。刘志鹏等通过冷休克诱导方法成功诱导出半滑舌鳎三倍体,发现雌雄ZWW/ZZZ核型出现的频率为1:1,雌性性别比例并没有显著提高,同时在诱导的三倍体中没有发现ZZW型雌鱼[92-93]。李文龙用静水压力诱导了半滑舌鳎三倍体和四倍体,,研究发现三倍体同二倍体相比,性腺发育会出现明显的受阻现象,性别鉴定发现,三倍体中雌鱼的比例仅为11%,雌性率显著低于正常二倍体群体,三倍体生长速度和二倍体没有显著差异[94]。为何不同处理组的三倍体在雌雄比例上存在较大差异,有待进一步探讨,但已有的研究成果表明,通过诱导多倍体提高半滑舌鳎的雌性率或者实现性别控制育种的方法也是不可行的。
4 总结与展望半滑舌鳎的雌雄差异及其性染色体的存在,使其成为性别决定、性腺分化和性别控制育种的优选研究对象。在水产养殖中雌性个体生长快、个体大,养殖效益高,有效提高雌性比例是水产养殖企业所祈求的。然而,在苗种生产过程中,为了保证苗种成活率和生长速度,生产企业往往采用较高的水温,这就造成了苗种中高比例的雌鱼发生性逆转,成为生理雄鱼。为了解决雌性苗种偏少,培育全雌苗种问题,国内多个团队开展了多年的努力,包括传统的细胞工程育种如雌核发育和多倍体诱导,通过筛选伪雄鱼进行杂交育种,通过基因编辑等手段,根据已有的研究成果发现,相比XY型性染色的物种,传统的育种方法尤其是细胞工程育种,对半滑舌鳎的性别控制育种并不适用,通过伪雄鱼进行杂交的方法也无法达到理想的目的,原因是半滑舌鳎性染色体分化较大,ZW伪雄鱼无法生产W型精子,雌核发育和伪雄鱼杂交均无法获得理想中的WW超雌鱼,因此,要通过超雌鱼培育全雌品种的路径是不可行的。
近年来伴随新技术的开发和应用,尤其是基因编辑技术的发展,该技术在模式动植物中展现出显著的优势,在作物性状改良方面效果显著,在水生动物中也得到应用,尤其是在淡水生物中,相关研究工作也广泛展开。受制于海水物种的特殊性,基因编辑技术在海洋动物中的应用相对滞后。在最近的研究中,Cui等通过TALEN技术对半滑舌鳎进行了基因编辑,运用该技术成功敲除Dmrt1基因,敲除Dmrt1基因的遗传雄性个体表现出和雌性个体相似的表型[18]。但怎样利用基因编辑技术在半滑舌鳎上实现性别控制育种,提高雌性比例,还有待大量的研究。
已有全基因组关联分析研究证实,半滑舌鳎中存在对环境敏感的SNP位点[59-60],携有敏感位点的雌雄个体容易受到高温等环境因素诱导产生的性逆转,从而减少养殖群体中生理雌性个体的比例,降低养殖效益。因此可以预期,利用分子筛选技术,在养殖苗种培育前剔除带有敏感SNP位点的亲本,保留对温度不敏感的亲本,虽然该方法无法获得全雌群体,但理论上可以有效地减少人工苗种中伪雄鱼的出现比例,提高养殖群体中生理雌鱼的比例,提高养殖效益,因此,这可能成为半滑舌鳎性别控制育种的有效手段和新途径。
| [1] |
Devlin R H, Nagahama Y. Sex determination and sex differentiation in fish: An overview of genetic, physiological, and environmental influences[J]. Aquaculture, 2002, 208: 191-364. DOI:10.1016/S0044-8486(02)00057-1
(  0) 0) |
| [2] |
Ospina-álvarez N, Piferrer F. Temperature-dependent sex determination in fish revisited: Prevalence, a single sex ratio response pattern, and possible effects of climate change[J]. PloS One, 2008, 3: e2837. DOI:10.1371/journal.pone.0002837
(  0) 0) |
| [3] |
Zhang Q, Sun X, Qi J, et al. Sex determination mechanisms in fish[J]. Journal of Ocean University of China, 2009, 8: 155-160. DOI:10.1007/s11802-009-0155-0
(  0) 0) |
| [4] |
Kamiya T, Kai W, Tasumi S, et al. A trans-species missense SNP in Amhr2 is associated with sex determination in the tiger pufferfish, Takifugu rubripes (Fugu)[J]. PLoS Genetics, 2012, 8: e1002798. DOI:10.1371/journal.pgen.1002798
(  0) 0) |
| [5] |
Li M, Sun Y, Zhao J, et al. A tandem duplicate of anti-müllerian hormone with a missense SNP on the Y chromosome is essential for male sex determination in Nile tilapia, Oreochromis niloticus[J]. PLoS Genetics, 2015, 11: e1005678. DOI:10.1371/journal.pgen.1005678
(  0) 0) |
| [6] |
Matsuda M, Nagahama Y, Shinomiya A, et al. DMY is a Y-specific DM-domain gene required for male development in the medaka fish[J]. Nature, 2002, 417: 559-563. DOI:10.1038/nature751
(  0) 0) |
| [7] |
Matsuda M, Shinomiya A, Kinoshita M, et al. DMY gene induces male development in genetically female (XX) medaka fish[J]. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104: 3865-3870. DOI:10.1073/pnas.0611707104
(  0) 0) |
| [8] |
Shao C, Li Q, Chen S, et al. Epigenetic modification and inheritance in sexual reversal of fish[J]. Genome Research, 2014, 24: 604-615. DOI:10.1101/gr.162172.113
(  0) 0) |
| [9] |
Yano A, Guyomard R, Nicol B, et al. An Immune-related gene evolved into the master sex-determining gene in rainbow trout, Oncorhynchus mykiss[J]. Current Biology, 2012, 22: 1423-1428. DOI:10.1016/j.cub.2012.05.045
(  0) 0) |
| [10] |
Sun Y, Yu H, Zhang Q, et al. Molecular characterization and expression pattern of two zona pellucida genes in half-smooth tongue sole (Cynoglossus semilaevis)[J]. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2010, 155: 316-321. DOI:10.1016/j.cbpb.2009.11.016
(  0) 0) |
| [11] |
姜言伟, 万瑞景. 渤海半滑舌鳎的生殖习性及产卵生态的研究[J]. 渔业科学进展, 1988, 9: 185-192. Jiang Y, Wan R. Reproductive behavior and spawning ecology of Cynoglossus semilaevis Gunther in the Bohai Sea[J]. Marine Fisheries Research, 1988, 9: 185-192. (  0) 0) |
| [12] |
周丽青, 杨爱国, 柳学周, 等. 半滑舌鳎染色体核型分析[J]. 水产学报, 2005, 29(3): 417-419. Zhou L, Yang A, Liu X, et al. The karyotype of the tonguefish Cynoglossus semilaevis[J]. Journal of Fisheries of China, 2005, 29(3): 417-419. (  0) 0) |
| [13] |
Wang X, Jiang J, Gao J, et al. Identification of two novel female-specific DNA sequences in half-smooth tongue sole, Cynoglossus semilaevis[J]. Aquaculture, 2013, 88-391: 49-53.
(  0) 0) |
| [14] |
Wang X, Zhang Q, Ren J, et al. The preparation of sex-chromosome-specific painting probes and construction of sex chromosome DNA library in half-smooth tongue sole (Cynoglossus semilaevis)[J]. Aquaculture, 2009, 297: 78-84. DOI:10.1016/j.aquaculture.2009.09.020
(  0) 0) |
| [15] |
Struussmann C A, Moriyama S, Hanke E F, et al. Evidence of thermolabile sex determination in pejerrey[J]. Journal of Fish Biology, 1996, 48: 643-651.
(  0) 0) |
| [16] |
Myosho T, Otake H, Masuyama H, et al. Tracing the emergence of a novel sex-determining Gene in medaka, Oryzias luzonensis[J]. Genetics, 2012, 191: 163-170. DOI:10.1534/genetics.111.137497
(  0) 0) |
| [17] |
Takehana Y, Matsuda M, Myosho T, et al. Co-option of Sox3 as the male-determining factor on the Y chromosome in the fish Oryzias dancena[J]. Nature Communication, 2014, 5: 4157. DOI:10.1038/ncomms5157
(  0) 0) |
| [18] |
Cui Z, Liu Y, Wang W, et al. Genome editing reveals dmrt1 as an essential male sex-determining gene in Chinese tongue sole (Cynoglossus semilaevis)[J]. Scientific Reports, 2017, 7: 42213. DOI:10.1038/srep42213
(  0) 0) |
| [19] |
Bao L, Tian C, Liu S, et al. The Y chromosome sequence of the channel catfish suggests novel sex determination mechanisms in teleost fish[J]. BMC Biology, 2019, 17: 6. DOI:10.1186/s12915-019-0627-7
(  0) 0) |
| [20] |
Deng S, Chen S. cDNA cloning, tissues, embryos and larvae expression analysis of Sox10 in half-smooth tongue sole, Cynoglossus semilaevis[J]. Marine Genomics, 2008, 1: 109-114. DOI:10.1016/j.margen.2008.10.003
(  0) 0) |
| [21] |
Deng S, Chen S, Xu J, et al. Molecular cloning, characterization and expression analysis of gonadal P450 aromatase in the half-smooth tongue sole, Cynoglossus semilaevis[J]. Aquaculture, 2009, 287: 211-218. DOI:10.1016/j.aquaculture.2008.10.034
(  0) 0) |
| [22] |
Liu J, Liu T, Niu J, et al. Expression pattern and functional analysis of R-spondin1 in tongue sole Cynoglossus semilaevis[J]. Gene, 2018, 642: 453-640. DOI:10.1016/j.gene.2017.11.047
(  0) 0) |
| [23] |
Liu J, Zhang W, Du X, et al. Molecular characterization and functional analysis of the GATA4 in tongue sole (Cynoglossus semilaevis)[J]. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2016, 193: 1-8. DOI:10.1016/j.cbpb.2015.12.001
(  0) 0) |
| [24] |
Liu J, Zhang W, Sun Y, et al. Molecular characterization and expression profiles of GATA6 in tongue sole (Cynoglossus semilaevis)[J]. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2016, 198: 19-26. DOI:10.1016/j.cbpb.2016.03.006
(  0) 0) |
| [25] |
Liu Y, Zhu H, Liu Y, et al. Molecular characterization and expression profiles provide new insights into GATA5 functions in tongue sole (Cynoglossus semilaevis)[J]. Gene, 2019, 708: 21-29. DOI:10.1016/j.gene.2019.05.024
(  0) 0) |
| [26] |
Sun Y, Zhang Q, Qi J, et al. Identification of differential genes in the ovary relative to the testis and their expression patterns in half-smooth tongue sole (Cynoglossus semilaevis)[J]. Journal of Genetics & Genomics, 2010, 37: 137-145.
(  0) 0) |
| [27] |
Wang K, Zhang H, Hu Q, et al. Expression and purification of half-smooth tongue sole (Cynoglossus semilaevis) CSDAZL protein[J]. Protein Expression and Purification, 2014, 102: 8-12. DOI:10.1016/j.pep.2014.07.006
(  0) 0) |
| [28] |
Wang W, Yi Q, Ma L, et al. Sequencing and characterization of the transcriptome of half-smooth tongue sole (Cynoglossus semilaevis)[J]. BMC Genomics, 2014, 15: 470. DOI:10.1186/1471-2164-15-470
(  0) 0) |
| [29] |
Chen S, Zhang G, Shao C, et al. Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle[J]. Nature Genetics, 2014, 46: 253-260. DOI:10.1038/ng.2890
(  0) 0) |
| [30] |
Aoyama S, Shibata K, Tokunaga S, et al. Expression of Dmrt1 protein in developing and in sex-reversed gonads of amphibians[J]. Cytogenetic and Genome Research, 2003, 101: 295-301. DOI:10.1159/000074352
(  0) 0) |
| [31] |
Guan G, Kobayashi T, Nagahama Y. Sexually dimorphic expression of two types of DM (Doublesex/Mab-3)-domain genes in a teleost fish, the tilapia (Oreochromis niloticus)[J]. Biochemical and Biophysical Research Communications, 2000, 272: 662-666. DOI:10.1006/bbrc.2000.2840
(  0) 0) |
| [32] |
Marchand O, Govoroun M, D'Cotta H, et al. DMRT1 expression during gonadal differentiation and spermatogenesis in the rainbow trout, Oncorhynchus mykiss[J]. Biochimica et Biophysica Acta (BBA) -Gene Structure and Expression, 2000, 1493: 180-187. DOI:10.1016/S0167-4781(00)00186-X
(  0) 0) |
| [33] |
Murdock C, Wibbels T. Expression of Dmrt1 in a turtle with temperature-dependent sex determination[J]. Cytogenetic and Genome Research, 2003, 101(3-4): 302-308. DOI:10.1159/000074353
(  0) 0) |
| [34] |
Nanda I, Kondo M, Hornung U, et al. A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes[J]. Proceedings of the National Academy of Sciences of the United States of America, 2002, 99: 11778-11783. DOI:10.1073/pnas.182314699
(  0) 0) |
| [35] |
Raymond C S, Kettlewell J R, Hirsch B, et al. Expression of Dmrt1 in the genital ridge of mouse and chicken embryos suggests a role in vertebrate sexual development[J]. Developmental Biology, 1999, 215: 208-220. DOI:10.1006/dbio.1999.9461
(  0) 0) |
| [36] |
Alam M A, Kobayashi Y, Horiguchi R, et al. Molecular cloning and quantitative expression of sexually dimorphic markers Dmrt1 and Foxl2 during female-to-male sex change in Epinephelus merra[J]. General and Comparative Endocrinology, 2008, 157: 75-85. DOI:10.1016/j.ygcen.2008.03.018
(  0) 0) |
| [37] |
Cao M, Duan J, Cheng N, et al. Sexually dimorphic and ontogenetic expression of dmrt1, cyp19a1a and cyp19a1b in Gobiocypris rarus[J]. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 2012, 162: 303-309.
(  0) 0) |
| [38] |
Jørgensen A, Morthorst J E, Andersen O, et al. Expression profiles for six zebrafish genes during gonadal sex differentiation[J]. Reproductive biology and endocrinology: RB&E, 2008, 6: 25-33.
(  0) 0) |
| [39] |
Ma L, Wang W, Yang X, et al. Characterization of the Dmrt1 gene in the black rockfish Sebastes schlegeli revealed a remarkable sex-dimorphic expression[J]. Fish Physiology and Biochemistry, 2014, 40: 1263-1274.
(  0) 0) |
| [40] |
Raghuveer K, Senthilkumaran B. Identification of multiple dmrt1s in catfish: Localization, dimorphic expression pattern, changes during testicular cycle and after methyltestosterone treatment[J]. Journal of Molecular Endocrinology, 2009, 42: 437-448. DOI:10.1677/JME-09-0011
(  0) 0) |
| [41] |
Shin H S, An K W, Park M S, et al. Quantitative mRNA expression of sox3 and DMRT1 during sex reversal, and expression profiles after GnRHa administration in black porgy, Acanthopagrus schlegeli[J]. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2009, 154: 150-156. DOI:10.1016/j.cbpb.2009.05.013
(  0) 0) |
| [42] |
Xia W, Zhou L, Yao B, et al. Differential and spermatogenic cell-specific expression of DMRT1 during sex reversal in protogynous hermaphroditic groupers[J]. Molecular and Cellular Endocrinology, 2007, 263: 156-172. DOI:10.1016/j.mce.2006.09.014
(  0) 0) |
| [43] |
Yamaguchi A, Lee K H, Fujimoto H, et al. Expression of the DMRT gene and its roles in early gonadal development of the Japanese pufferfish Takifugu rubripes[J]. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics, 2006, 1(1): 59-68. DOI:10.1016/j.cbd.2005.08.003
(  0) 0) |
| [44] |
李林, 梁宏伟, 李忠, 等. 黄颡鱼DMRT1基因cDNA全长克隆及其表达分析[J]. 华中农业大学学报, 2012, 31: 220-226. Li L, Liang H, Li Z, et al. Cloning and expression analysis of DMRT1 gene in Pelteobagrus fulvidraco[J]. Journal of Huazhong Agricultural University, 2012, 31: 220-226. DOI:10.3969/j.issn.1000-2421.2012.02.017 (  0) 0) |
| [45] |
文爱韵, 尤锋, 孙鹏, 等. 牙鲆dmrt1基因的克隆及其与P450arom基因的组织表达分析[J]. 海洋科学, 2010, 34: 97-102. Wen A, You F, Sun P, et al. Cloning of dmrt1 gene and its tissue expression analyses compared with that of P450arom gene in olive flounder (Paralichthys olivaceus)[J]. Marine Sciences, 2010, 34: 97-102. (  0) 0) |
| [46] |
Yoshimoto S, Ikeda N, Izutsu Y, et al. Opposite roles of DMRT1 and its W-linked paralogue, DM-W, in sexual dimorphism of Xenopus laevis: Implications of a ZZ/ZW-type sex-determining system[J]. Development, 2010, 137: 2519. DOI:10.1242/dev.048751
(  0) 0) |
| [47] |
Yoshimoto S, Okada E, Umemoto H, et al. A W-linked DM-domain gene, DM-W, participates in primary ovary development in Xenopus laevis[J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105: 2469-2474. DOI:10.1073/pnas.0712244105
(  0) 0) |
| [48] |
邓思平.半滑舌鳎性别相关基因P450芳香化酶、FTZ-F1和DMRT1基因克隆及表达分析[D].青岛: 中国海洋大学, 2007. Deng S. Molecular Cloning, Characterization and Expression Analysis of Sex-Related Gene of P450aromatase, FTZ-F1 and DMRT1 in Half-Smooth Tongue Sole, Cynoglossus semilaevis Gunther[D]. Qingdao: Ocean University of China, 2007. (  0) 0) |
| [49] |
孙业盈, 张全启, 齐洁, 等. 半滑舌鳎DMRT1基因的克隆与表达分析[J]. 武汉大学学报(理学版), 2008, 221-226. Sun Y, Zhang Q, Qi J, et al. Cloning and expression analysis of DMRT1 gene in Cynoglossus semilaevis[J]. Journal of Wuhan Universit (Natural Science Edition), 2008, 221-226. DOI:10.3321/j.issn:1671-8836.2008.02.019 (  0) 0) |
| [50] |
Conover D O, Kynard B E. Environmental sex determination: Interaction of temperature and genotype in a fish[J]. Science, 1981, 213: 577-579. DOI:10.1126/science.213.4507.577
(  0) 0) |
| [51] |
Baroiller J F, D'Cotta H. Environment and sex determination in farmed fish[J]. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 2001, 130: 399-409.
(  0) 0) |
| [52] |
Liu J, Liu X, Jin C, et al. Transcriptome profiling insights the feature of sex reversal induced by high temperature in tongue sole Cynoglossus semilaevis[J]. Frontiers in Genetics, 2019, 10: 522. DOI:10.3389/fgene.2019.00522
(  0) 0) |
| [53] |
季相山, 陈松林, 马洪雨, 等. 半滑舌鳎养殖群体中自然性逆转伪雄鱼的发现[J]. 水产学报, 2010, 34: 322-327. Ji X, Chen S, Ma H, et al. Natural sex reversal of female in Cynoglossus semilaevis rearing populaitons[J]. Journal of Fisheries of China, 2010, 34: 322-327. (  0) 0) |
| [54] |
王旭波.半滑舌鳎(Cynoglossus semilaevis)雌鱼分子细胞遗传学分析[D].青岛: 中国海洋大学, 2008. Wang X. Molecular Cytogenetic Analysis in Female Half-Smooth Tongue Sole (Cynoglossus semilaevis)[D]. Qingdao: Ocean University of China, 2008. (  0) 0) |
| [55] |
Chen S, Ji X, Shao C, et al. Induction of mitogynogenetic diploids and identification of WW super-female using sex-specific SSR markers in half-smooth tongue sole (Cynoglossus semilaevis)[J]. Marine Biotechnology, 2012, 14: 120-128. DOI:10.1007/s10126-011-9395-2
(  0) 0) |
| [56] |
Liao X, Ma H, Xu G, et al. Construction of a genetic linkage map and mapping of a female-specific DNA marker in half-smooth tongue sole (Cynoglossus semilaevis)[J]. Marine Biotechnology, 2009, 11: 699. DOI:10.1007/s10126-009-9184-3
(  0) 0) |
| [57] |
Zhang L, Liu W, Shao C, et al. Cloning, expression and methylation analysis of piwil2 in half-smooth tongue sole (Cynoglossus semilaevis)[J]. Marine Genomics, 2014, 18: 45-54. DOI:10.1016/j.margen.2014.04.004
(  0) 0) |
| [58] |
张全启, 王旭波, 王志刚, 等.牙鲆和半滑舌鳎性别控制育种研究进展[C].//南京: "全球变化下的海洋与湖沼生态安全"学术交流会, 2014. Zhang Q, Wang X, Wang Z, et al. The prograss of sex control breeding in Japanese flounder and half smooth tongue sole[C].//Nanjing: Symposium of Marine and Lake Ecosystem Safety Under Global Change, 2014. (  0) 0) |
| [59] |
Jiang L, Li H. Single locus maintains large variation of sex reversal in half-smooth tongue sole (Cynoglossus semilaevis)[J]. G3: Genes Genomes Genetics, 2017, 7: 583-589.
(  0) 0) |
| [60] |
Cui Y, Wang W, Ma L, et al. New locus reveals the genetic architecture of sex reversal in the Chinese tongue sole (Cynoglossus semilaevis)[J]. Heredity, 2018, 121: 319-326. DOI:10.1038/s41437-018-0126-6
(  0) 0) |
| [61] |
Flament S. Sex reversal in amphibians[J]. Sexual Development, 2016, 10: 267-278. DOI:10.1159/000448797
(  0) 0) |
| [62] |
Sarre S D, Ezaz T, Georges A. Transitions between sex-determining systems in reptiles and amphibians[J]. Annual Review of Genomics and Human Genetics, 2011, 12: 391-406. DOI:10.1146/annurev-genom-082410-101518
(  0) 0) |
| [63] |
Fernandino J I, Hattori R S, Moreno Acosta O D, et al. Environmental stress-induced testis differentiation: Androgen as a by-product of cortisol inactivation[J]. General and Comparative Endocrinology, 2013, 192: 36-44. DOI:10.1016/j.ygcen.2013.05.024
(  0) 0) |
| [64] |
Fernandino J I, Somoza G M, Kishii A, et al. The cortisol and androgen pathways cross talk in high temperature-induced masculinization: The 11β-hydroxysteroid dehydrogenase as a key enzyme[J]. Endocrinology, 2012, 153: 6003-6011. DOI:10.1210/en.2012-1517
(  0) 0) |
| [65] |
Hattori R S, Fernandino J I, Kishii A, et al. Cortisol-induced masculinization: Does thermal stress affect gonadal fate in pejerrey, a teleost fish with temperature-dependent sex determination?[J]. PloS One, 2009, 4: e6548. DOI:10.1371/journal.pone.0006548
(  0) 0) |
| [66] |
Kitano T, Hayashi Y, Shiraishi E, et al. Estrogen rescues masculinization of genetically female medaka by exposure to cortisol or high temperature[J]. Molecular Reproduction and Development, 2012, 79: 719-726. DOI:10.1002/mrd.22080
(  0) 0) |
| [67] |
Lance VA. Is regulation of aromatase expression in reptiles the key to understanding temperature-dependent sex determination?[J]. Journal of Experimental Zoology Part A: Ecological Genetics and Physiology, 2009, 311A: 314-322. DOI:10.1002/jez.465
(  0) 0) |
| [68] |
Nakamura M. The mechanism of sex determination in vertebrates-are sex steroids the key-factor?[J]. Journal of Experimental Zoology Part A: Ecological Genetics and Physiology, 2010, 313A: 381-398. DOI:10.1002/jez.616
(  0) 0) |
| [69] |
Navarro-Martín L, Vias J, Ribas L, et al. DNA methylation of the gonadal aromatase (cyp19a) promoter is involved in temperature-dependent sex ratio shifts in the European sea bass[J]. PLoS Genetics, 2011, 7: e1002447. DOI:10.1371/journal.pgen.1002447
(  0) 0) |
| [70] |
Piferrer F. Epigenetics of sex determination and gonadogenesis[J]. Developmental Dynamics, 2013, 242: 360-370. DOI:10.1002/dvdy.23924
(  0) 0) |
| [71] |
Yoshinaga N, Yamaguchi T, Kitano T, et al. Cortisol is involved in temperature-dependent sex determination in the Japanese flounder[J]. Endocrinology, 2010, 151: 3900-3908. DOI:10.1210/en.2010-0228
(  0) 0) |
| [72] |
Deveson I W, Holleley C E, Blackburn J, et al. Differential intron retention in Jumonji chromatin modifier genes is implicated in reptile temperature-dependent sex determination[J]. Science Advances, 2017, 3: e1700731. DOI:10.1126/sciadv.1700731
(  0) 0) |
| [73] |
Matsumoto Y, Hannigan B, Crews D. Temperature shift alters DNA methylation and histone modification patterns in gonadal aromatase (cyp19a1) gene in species with temperature-dependent sex determination[J]. PloS One, 2016, 11: e0167362. DOI:10.1371/journal.pone.0167362
(  0) 0) |
| [74] |
Pieau C, Dorizzi M. Oestrogens and temperature-dependent sex determination in reptiles: All is in the gonads[J]. Journal of Endocrinology, 2004, 181: 367-377. DOI:10.1677/joe.0.1810367
(  0) 0) |
| [75] |
Valdivia K, Jouanno E, Volff J, et al. High temperature increases the masculinization rate of the all-female (XX) rainbow trout "Mal" population[J]. PloS One, 2014, 9: e113355. DOI:10.1371/journal.pone.0113355
(  0) 0) |
| [76] |
Wang Y, Sun L, Zhu J, et al. Epigenetic control of cyp19a1a expression is critical for high temperature induced Nile tilapia masculinization[J]. Journal of Thermal Biology, 2017, 69: 76-84. DOI:10.1016/j.jtherbio.2017.06.006
(  0) 0) |
| [77] |
Si Y, Ding Y, He F, et al. DNA methylation level of cyp19a1a and Foxl2 gene related to their expression patterns and reproduction traits during ovary development stages of Japanese flounder (Paralichthys olivaceus)[J]. Gene, 2016, 575: 321-530. DOI:10.1016/j.gene.2015.09.006
(  0) 0) |
| [78] |
Bizuayehu T, Johansen S, Puvanendran V, et al. Temperature during early development has long-term effects on microRNA expression in Atlantic cod[J]. BMC Genomics, 2015, 16: 305. DOI:10.1186/s12864-015-1503-7
(  0) 0) |
| [79] |
Czerwinski M, Natarajan A, Barske L, et al. A timecourse analysis of systemic and gonadal effects of temperature on sexual development of the red-eared slider turtle Trachemys scripta elegans[J]. Developmental Biology, 2016, 420: 166-177. DOI:10.1016/j.ydbio.2016.09.018
(  0) 0) |
| [80] |
Kohno S, Katsu Y, Urushitani H, et al. Potential contributions of heat shock proteins to temperature-dependent sex determination in the American alligator[J]. Sexual Development, 2010, 4: 73-87. DOI:10.1159/000260374
(  0) 0) |
| [81] |
Rhen T, Schroeder A. Molecular mechanisms of sex determination in reptiles[J]. Sexual Development, 2010, 4: 16-28. DOI:10.1159/000282495
(  0) 0) |
| [82] |
Schroeder A, Metzger K, Miller A, et al. A novel candidate gene for temperature-dependent sex determination in the common snapping turtle[J]. Genetics, 2016, 203: 557. DOI:10.1534/genetics.115.182840
(  0) 0) |
| [83] |
Domingos J, Budd A, Banh Q, et al. Sex-specific dmrt1 and cyp19a1 methylation and alternative splicing in gonads of the protandrous hermaphrodite barramundi[J]. PloS One, 2018, 13: e0204182. DOI:10.1371/journal.pone.0204182
(  0) 0) |
| [84] |
Du X, Liu X, Zhang K, et al. Discovery and functional characterization of microRNAs and their potential roles for gonadal development in spotted knifejaw, Oplegnathus punctatus[J]. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics, 2018, 28: 1-8. DOI:10.1016/j.cbd.2018.05.002
(  0) 0) |
| [85] |
Jing J, Wu J, Liu W, et al. Sex-biased miRNAs in gonad and their potential roles for testis development in yellow catfish[J]. PloS One, 2014, 9: e107946. DOI:10.1371/journal.pone.0107946
(  0) 0) |
| [86] |
Liu J, Luo M, Sheng Y, et al. Dynamic evolution and biogenesis of small RNAs during sex reversal[J]. Scientific Reports, 2015, 5: 9999. DOI:10.1038/srep09999
(  0) 0) |
| [87] |
Song H, Xing C, Lu W, et al. Rapid evolution of piRNA pathway and its transposon targets in Japanese flounder (Paralichthys olivaceus)[J]. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics, 2019, 100609.
(  0) 0) |
| [88] |
戈文龙, 张全启, 齐洁, 等. 异源精子诱导牙鲆雌核发育二倍体[J]. 中国海洋大学学报(自然科学版), 2005, 35: 1011-1016. Ge W, Zhang Q, Qi J, et al. Gynogenesis induced by heterogenous sperms and cold shock in olive flounder Paralichthys olivaceus[J]. Periodical of Ocean University of China, 2005, 35: 1011-1016. (  0) 0) |
| [89] |
Arai K. Genetic improvement of aquaculture finfish species by chromosome manipulation techniques in Japan[J]. Aquaculture, 2001, 197: 205-228. DOI:10.1016/S0044-8486(01)00588-9
(  0) 0) |
| [90] |
Guo X, DeBrosse G, Allen S. All-triploid Pacific oysters (Crassostrea gigas Thunberg) produced by mating tetraploids and diploids[J][J]. Aquaculture, 1996, 142: 149-161. (  0) 0) |
| [91] |
Piferrer F, Beaumont A, Falguière J, et al. Polyploid fish and shellfish: Production, biology and applications to aquaculture for performance improvement and genetic containment[J]. Aquaculture, 2009, 293: 125-156. DOI:10.1016/j.aquaculture.2009.04.036
(  0) 0) |
| [92] |
陈松林, 李文龙, 季相山, 等. 半滑舌鳎三倍体鱼苗的人工诱导与鉴定[J]. 水产学报, 2011, 35: 925-931. Chen S, Li W, Ji X, et al. Induction and identification of artificial triploid fry in Cynoglossus semilaevis[J]. Journal of Fisheries of China, 2011, 35: 925-931. (  0) 0) |
| [93] |
刘志鹏, 王旭波, 翟介明, 等. 半滑舌鳎三倍体诱导研究[J]. 中国海洋大学学报(自然科学版), 2012, 42: 77-80. Liu Z, Wang X, Zhai J, et al. Study on triploid induction in the half-smooth tongue sole (Cynoglossus semilaevis)[J]. Periodical of Ocean Universityof of China, 2012, 42: 77-80. (  0) 0) |
| [94] |
李文龙.半滑舌鳎多倍体的人工诱导及三倍体生长与发育的研究[D].上海: 上海海洋大学, 2012. Li W. Studies on the Artificial Induction of Polyploidy and Its Effect on Growth and Development of Triploid in Half-Smooth Tongue Sole (Cynoglossus semilaevis)[D]. Shanghai: Shanghai Ocean University, 2012. (  0) 0) |
2. Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China
 2019, Vol. 49
2019, Vol. 49

