转录因子(Transcription factor,TF),也称反式作用因子,是指能够与真核基因的顺式作用元件发生特异性相互作用,以激活或抑制基因转录的DNA结合蛋白[1]。调节因子X(Regulatory Factor X, RFX)蛋白家族是一类转录因子,含高度保守的有翼螺旋型DNA结合域,能够特异性与基因启动子区的X盒(5’-GTNRCC(0-3N)RGYAAC-3’,其中N为任意核苷酸,R为嘌呤,Y为嘧啶)[2]结合。已有研究表明RFX蛋白家族不仅参与纤毛和精子发生、免疫防御以及细胞周期调控等多种生物学过程,也与肿瘤的增殖过程密切相关。本文主要对该蛋白家族的发现、各成员的结构特征、表达模式及生物学功能进行阐述。
1 RFX蛋白家族的发现1988年,RFX1首次在一种先天性重症联合免疫缺陷(SCID)疾病中被发现[3-4],之后RFX蛋白家族其他各成员也被逐渐分离鉴定出来,截止目前共发现8个RFX成员,分别是RFX1、RFX2、RFX3、RFX4、RFX5、RFX6、RFX7和RFX8。Reith等证明RFX1作为一种转录因子,间接参与乙型肝炎病毒(HBV)基因的表达调控,对HBV诱导的肝炎和肝癌的发病机制至关重要[5-7]。虽然RFX2和RFX3的结构与RFX1相似,但它们的功能和作用机制与RFX1存在差异[7-8]。1992年,Dotzlaw等[9]发现了人类乳腺癌的异常cDNA。其中这个新的RFX蛋白被认为是RFX蛋白家族的成员之一,后来称为RFX4[10]。1995年,Steimle等[11]发现RFX5,其突变可以导致MHC Ⅱ缺乏症或裸淋巴细胞综合征(BLS)。RFX6和RFX7于2008年被鉴定为新的人类RFX家族成员[12],随后发现RFX6与胰腺发育有关[13-14],是导致人类新生儿糖尿病的单致病基因[15]。RFX7则是一种新的自然杀伤细胞稳态和代谢途径的转录调节因子[16]。RFX8在2018年首次被报道[17],其功能尚不明确。
2 RFX蛋白家族的成员和结构 2.1 RFX蛋白家族成员动物和真菌中均存在RFX家族基因,而在植物和细菌中的分布尚未报道[18]。利用NCBI(https://www.ncbi.nlm.nih.gov/)和Ensembl(http://asia.ensembl.org/index.html)数据库检索并比较了人(Homo sapiens)、小鼠(Mus musculus)、鸡(Gallus gallus)、非洲爪蛙(Xenopus tropicalis)、斑马鱼(Danio rerio)、黑腹果蝇(Drosphila melanogaster)、白氏文昌鱼(Branchiostoma belcheri)、海鞘(Ciona intestinalis)、秀丽隐杆线虫(Caenorhabditis elegans)和酿酒酵母(Saccharomyces cerevisiae)10个模式生物中Rfx家族基因的拷贝数。如表 1所示,本研究中发现目前Rfx家族基因在哺乳类动物的人和小鼠中有8个成员(Rfx1、Rfx2、Rfx3、Rfx4、Rfx5、Rfx6、Rfx7和Rfx8);在属于鸟类的鸡中,Rfx1在基因组中并未找到,仅包括7个成员(Rfx2~8);两栖类动物非洲爪蛙中有7个成员(Rfx1、Rfx2、Rfx3、Rfx4、Rfx5、Rfx6和Rfx7)。而在硬骨鱼中,由于特有的多出的一次基因组复制,在斑马鱼中发现2个rfx1基因(rfx1a和rfx1b)和2个rfx7基因(rfx7a和rfx7b)。头索动物白氏文昌鱼、尾索动物玻璃海鞘以及无脊椎动物秀丽隐杆线虫和黑腹果蝇中该家族成员明显与脊椎动物中的不同,白氏文昌鱼中发现有一个Rfx同源基因,玻璃海鞘中发现有3个Rfx同源基因,秀丽隐杆线虫中仅有一个Rfx同源基因,黑腹果蝇中则有2个Rfx同源基因[19]。而在真菌酿酒酵母中有一个Rfx同源基因。不同进化地位代表性动物中某一基因家组成员个数的差别,表明该家族在高等动物中发生了基因扩增。
|
|
表 1 代表性模式生物中Rfx基因的拷贝数 Table 1 Copy number of Rfx genes in representative model organisms |
RFX家族成员的蛋白序列长度各不相同。各成员蛋白均含有一个高度保守的DNA结合域,除该结构域外,不同的RFX家族蛋白含有的结构域并不相同。以人为例,RFX1、RFX2和RFX3除DNA结合域之外,还包含RFX激活域、参与转录抑制的B域和C域以及介导同型或异型二聚体形成的二聚化结构域,而RFX4、RFX6和RFX8不具有RFX激活域,只具有DNA结合域、B域和C域以及二聚化结构域,更特殊的RFX5和RFX7仅包含DNA结合域[17](见图 1)。RFX蛋白在体外可以以单体的形式与DNA结合[20],但在体内它们更倾向于以同源二聚体或异源二聚体的形式结合[21-24]。
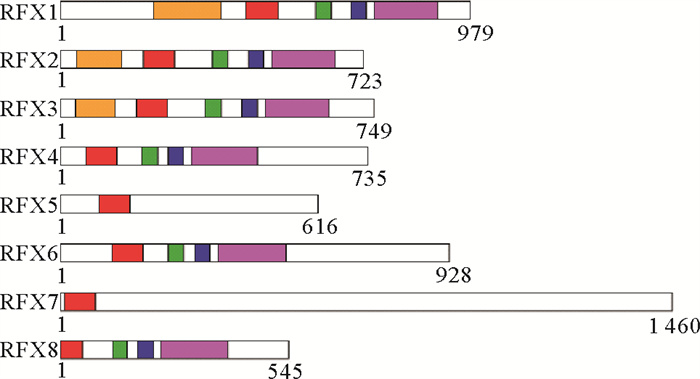
|
(橙色:RFX激活域;红色:有翼螺旋DNA结合域;绿色:B结构域;蓝色:C结构域;紫色:二聚化结构域,数字表示蛋白长度。Orange: RFX active domain; Red: DNA binding domain; Green: B domain; Blue: C domain; Purple: Dimerization domain. ) 图 1 人RFX蛋白家族成员结构域组成(改自文献[17] Fig. 1 Domain composition of human RFX family protein (Modified from Reference[17]) |
目前关于RFX家族成员的表达在mRNA水平呈现多样化的表达模式。在基于人类135份组织样本的研究中发现RFX1、RFX2、RFX3和RFX4主要在睾丸、大脑和脊髓中表达,另外也发现RFX2在子宫部分表达,RFX3在肺中有表达;RFX5在免疫相关组织中表达较高,其在胃肠道中也有表达;RFX6主要表达在胃肠道中;RFX7主要在大脑和脊髓中表达,而在这些组织中均未检测到RFX8的表达[17]。在非洲爪蛙胚胎发育中发现Rfx1、Rfx2、Rfx3、Rfx4和Rfx5均在神经管中有表达,值得注意的是Rfx2和Rfx3主要在含有纤毛的组织中表达,如上皮纤毛细胞和胃腔顶板(GRP),但Rfx3的表达弱于Rfx2的表达强度,Rfx4在中枢神经系统中高表达,但在胃腔顶板或表皮中却未检测到[25],非洲爪蛙其他Rfx成员的表达未见报道。斑马鱼中目前仅有rfx2、rfx4和rfx6的相关报道,rfx2的表达模式同非洲爪蛙Rfx2相似,也在纤毛相关组织中表达[26],rfx4则广泛表达于中脑、后脑和脊髓[27]中,而rfx6在胰腺中表达[14]。其他物种中Rfx成员的表达模式尚有待研究。而关于RFX蛋白水平的表达几乎没有报道,主要的原因是缺乏抗体。
4 RFX的生物学功能 4.1 RFX与纤毛发生RFX家族蛋白的重要功能之一是纤毛的发生和功能维持。纤毛存在于从原生动物到脊椎动物的绝大多数物种的细胞表面,纤毛基因突变会导致纤毛形成异常,进而使纤毛功能受损,引发疾病[28]。RFX蛋白家族参与纤毛发生的研究最早可追溯于秀丽隐杆线虫,Rfx同源基因daf-19特异表达于纤毛感觉神经元,其功能丧失导致纤毛的缺失,引发严重的感觉缺陷[29]。之后在黑腹果蝇中发现,同源基因drfx调控的许多基因与daf-19靶基因同源[30],这提示daf-19具有功能的保守性。
在脊椎动物中,RFX2和RFX3调控的纤毛发生对胚胎的早期发育至关重要[31-33]。Rfx 3基因敲除小鼠存在一定比例的死亡和生长迟缓现象,约6%的新生突变体幼鼠出现内脏器官左右分布完全倒置[34]。器官的左右不对称发育依赖于一类具有特殊功能的单纤毛所产生的Nodal液流(Node flow)[35]。在Rfx3突变体小鼠中这种单纤毛的长度明显短于野生型,是由于Rfx3调控纤毛组装和维护相关基因D2lic的表达所致[34]。很早就有证明Rfx2的表达受纤毛基因转录因子FoxJ1控制[36-37],缺失Rfx2的斑马鱼和爪蛙中会出现纤毛发育障碍所导致的胚胎左右体轴不对称发育缺陷[25-26]。
RFX蛋白家族其他成员也以不同机制参与纤毛发生。Rfx4的缺失能降低编码纤毛发生所必需的内转运蛋白基因Ift172的表达,通过纤毛发生受损从而影响中枢神经系统的发育[38]。Rfx7通过调控Rfx4基因的表达影响神经管纤毛的发生[39]。RFX1能调控人类纤毛基因ALMS1的表达[40],而ALMS1基因突变会诱发Alstrom综合征。但目前相关研究并未系统化,RFX家族一些成员的功能及精细调控机制有待深入。
4.2 RFX与精子发生精子发生是在睾丸的曲细精管中,该过程包括精原细胞的增殖和分化、精母细胞的减数分裂和精子细胞的变形。另外,精子发生过程中有许多睾丸特异基因进行表达,以产生新的酶类或其他蛋白大分子,从而调控精子发生[41]。哺乳动物RFX蛋白家族中RFX1~4在睾丸中均有表达[17, 42],而且在大规模筛选小鼠组织特异性的转录调控基序时发现,大量睾丸特异表达基因的启动子上包含丰富的RFX转录因子调控位点[43],这提示RFX转录因子可能在精子发生过程起到重要调控作用。
RFX2是精子发生过程中的一个重要调控因子,也是目前精子发生过程中研究最多最透彻的一个RFX家族蛋白成员。已证明它能直接调控几种睾丸特异性基因如H1t[44-46]和Alf[47]的表达,并且在敲除Rfx2基因的小鼠中,除大约25%的小鼠随着年龄增长出现严重的生长迟缓并存活期缩短外,其余的突变体小鼠表现出明显的雄性不育。进一步研究发现Rfx2缺失一方面导致圆形精子细胞发育停滞并伴有细胞凋亡,另一方面导致鞭毛相关基因表达下降, 影响鞭毛组装[48-49]。
其他RFX蛋白家族成员可能在精子发生过程中有作用,RFX1能调控精子发生相关4基因(Spata4)的表达[50],条件性敲除RFX1影响睾丸索发育进而导致精子发生过程受阻[51];RFX4在生殖细胞中可以通过与RFX2的结合阻碍RFX2自身同源二聚体的形成,从而有效阻止睾丸特异性组蛋白H1t基因在精母细胞中表达[46, 52]。该家族蛋白的其他成员在精子发生中是否起作用值得探究。
4.3 RFX与免疫部分RFX蛋白成员参与免疫应答。线虫的DAF-19能与转录因子ATF-7协同作用,上调抗菌基因tph-1的表达,参与先天免疫应答[53]。脊椎动物的RFX蛋白能通过调控MHCⅡ类基因的表达执行免疫功能。MHCⅡ类分子将处理过的抗原递呈给CD4+T细胞,参与机体免疫应答[54]。MHCⅡ类基因的表达受到RFX复合物和Ⅱ类转录激活因子(ClassⅡtransactivator,CⅡTA)的严格调控,RFX复合体的任意成员或是CⅡTA突变都会导致原发性免疫缺陷病即裸淋巴细胞综合征(BLS)的产生[55]。RFX5是RFX复合物的重要组成部分,含锚蛋白副本的RFXANK和RFX相关蛋白RFXAP共同组成的异源三聚体,招募X2BPs(X2 box Binding Proteins)和NF-Y(Nuclear Factor Y)形成MHCⅡ类基因增强体,MHCⅡ类基因增强体进一步与CⅡTA结合并招募其他调节因子促进MHCⅡ类基因的表达[56-58]。RFX7可限制体内自然杀伤(NK)细胞的代谢并促进其功能维持,RFX7缺失可导致机体免疫力下降[16]。同时,最近研究发现RFX7的两个区域可以分别同锚蛋白副本家族A蛋白(ANKRA2)和RFXANK结合[59],这同RFX5的作用机制有相似之处,而其是否调控MHCⅡ的表达有待验证。
RFX蛋白家族的另一成员RFX1会影响自身免疫性疾病发生,其下调能增加小鼠CD4+T细胞中白细胞介素(IL)17A表达并促进CD4+17型辅助T细胞(Th17)分化,进一步加重小鼠的脑脊髓炎和系统性红斑狼疮[60]。虽然RFX蛋白家族中有保守的能同MHCⅡ类基因启动子上的X盒结合的翼螺旋DNA结合域,但截止目前除RFX5、RFX7和RFX1外,并未发现其他成员参与免疫应答。
4.4 RFX与细胞周期及癌症真菌核分裂过程受到RFX因子的精确调控,裂殖酵母(Schizosaccharomyces pombe)和酿酒酵母(S.cerevisiae)中RFX表达的紊乱导致细胞生长和分化缺陷[61-64]。人RFX1调控增殖细胞核抗原(PCNA)基因的表达,而PCNA与DNA复制和细胞周期调控过程密切相关[65]。
细胞周期调控和肿瘤转化密不可分,细胞周期调控失调会导致肿瘤发生[66]。越来越多的研究表明RFX同多种癌症的发生、转移及预后过程有关。食管鳞状细胞癌、胃腺癌、结肠腺癌、直肠腺癌、肝癌和胰腺癌六种消化系统肿瘤组织中RFX5的mRNA和蛋白质水平均明显高于其他组织[67],且RFX5能结合同肝癌患者预后不良显著相关的三肽基肽酶1基因(TPP1)的启动子区,增强其转录活性[68]。另外,RFX1调控相关基因的表达从而抑制多种癌细胞的增殖,如RFX1能抑制转化生长因子β2基因(TGFβ2)的表达,从而抑制神经母细胞瘤细胞SH-SY5Y的增值[69-70]。Rfx1过表达能激活SHP-1,从而抑制肝细胞癌(HCC)细胞集落形成能力[71]。RFX4能维持胶质瘤干细胞(GSCs)干性,是胶质母细胞瘤(GBM)恶化的潜在原因,有望成为GBM治疗的靶点[72]。RFX7能抑制阿霉素诱导的细胞凋亡,是肿瘤细胞生长和命运决定的重要调节因子[73]。综上所述,RFX蛋白家族能通过调控肿瘤相关基因促进或抑制肿瘤的增殖,探究RFX蛋白家族各成员在肿瘤发展进程中的机制对肿瘤的预防和治疗具有重要意义。
5 总结与展望本文就RFX蛋白家族的发现、各成员的结构特征、表达模式及生物学功能进行综述。RFX蛋白家族是一类重要的转录因子,在不同生物过程中执行多样化的功能。该家族成员的表达模式呈现多样化的表达模式,这同其功能的多样化是吻合的。此外,该家族的不同成员之间表达既有相似也有不同,在不同进化地位的模式动物中RFX同源基因的表达呈现出一定的相似性,这表明无论是同一物种的不同RFX成员,还是在不同物种的同源基因的功能可能既具保守性也有差异,提示RFX家族成员功能的复杂性。RFX家族成员蛋白的结构域具有一定的保守性,除高度保守的DNA结合域外,不同蛋白成员包含的结构域有一定差异,这可能是不同成员的功能既有相似又有很大差异的原因。截止目前,该家族蛋白成员主要作为转录因子调控靶基因的表达,它们直接与基因启动子上的X盒结合,也可以先与其他转录因子结合形成复合物后再与基因启动子结合,而后可能会招募更多的调节因子来促进或抑制基因的表达,进而调控生物发生过程(见图 2)。
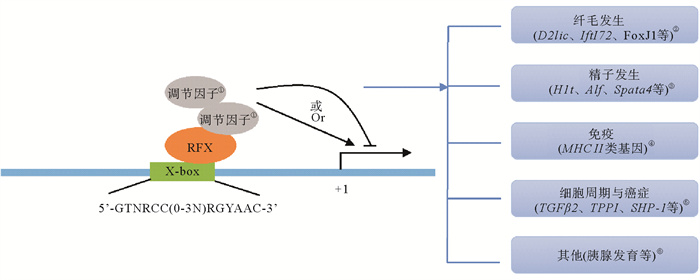
|
(①Regulatory factors;②Ciliogenesis (D2lic, Ift172, FoxJ1, etc.);③Spernatogenesis (H1t, Alf, Spata4, etc.);④Immunity (MHCⅡ genes);⑤Cell Cycle and Cancer (TGFβ2, TPP1, SHP-1, etc.);⑥Others (Pancreatic development, etc.). RFX蛋白结合相关基因启动子上的X-box,并可能招募数量不等的调节因子激活或抑制基因的表达,进而调控不同生物过程。在X-box序列中,N为任意核苷酸,R为嘌呤, Y为嘧啶。RFX proteins bind to X-boxes on promoters of relevant genes and may recruit varying numbers of regulatory factors to activate or inhibit gene expression, thereby regulating different biological processes. In X-box sequence, N is any nucleotide, R is purine, and Y is pyrimidine. ) 图 2 RFX调控模式图 Fig. 2 RFX regulation pattern diagram |
RFX蛋白家族不同成员既具功能冗余性又有各自的独特性,从而在多种生物过程中起着精细的调控作用。一个成员缺失后生物体会表现出复杂的功能代偿,如RFX3的缺失导致大脑中一些多纤毛细胞类型的纤毛数量减少,但其他细胞类型的纤毛数量增加[74],而在小鼠离体分化的室管膜细胞中,RFX2和RFX3双缺失会导致室管膜细胞纤毛发生显著损伤,而单独缺失并不会影响细胞纤毛的数量和形态[75]。这种功能冗余和代偿有利于机体应对环境突发事件,是机体必不可少的[76]。在斑马鱼中敲除Rfx6基因会导致胰岛细胞的发育异常和胰岛素的水平下降[77],Rfx1/3双敲除的小鼠品系由于外耳毛细胞的晚期分化异常导致听力丧失[78],这些研究成果提示RFX家族成员参与的生物过程更为广泛,RFX蛋白家族不同成员在体内的具体作用以及彼此之间的相互关系值得深入探讨。RFX蛋白家族成员同肿瘤发生有关,也同某些恶性肿瘤的进程和预后不良相关,提示RFX蛋白家族成员有望成为癌症治疗的新靶点。尽管关于RFX蛋白家族已有很多研究,但目前的研究远远不足,有待深入探索。
| [1] |
Rodriguez-Caso C, Medina M A, Solé R. Topology, tinkering and evolution of the human transcription factor network[J]. FEBS Journal, 2010, 272(24): 6423-6434. (  0) 0) |
| [2] |
Tsang S Y, Nakanishi M, Peterlin B M. Mutational analysis of the DRA promoter: Cis-acting sequences and trans-acting factors[J]. Molecular & Cellular Biology, 1990, 10(2): 711-719. (  0) 0) |
| [3] |
Reith W, Barras E, Satola S, et al. Cloning of the major histocompatibility complex classⅡ promoter binding protein affected in a hereditary defect in classⅡ gene regulation[J]. Proceedings of The National Academy of Sciences of the United States of America, 1989, 86(11): 4200-4204. DOI:10.1073/pnas.86.11.4200 (  0) 0) |
| [4] |
Reith W, Satola S, Sanchez C H, et al. Congenital immunodeficiency with a regulatory defect in MHC class Ⅱ gene expression lacks a specific HLA-DR promoter binding protein, RF-X[J]. Cell, 1988, 53(6): 897-906. DOI:10.1016/S0092-8674(88)90389-3 (  0) 0) |
| [5] |
Ostapchuk P, Scheirle G, Hearing P. Binding of nuclear factor EF-C to a functional domain of the hepatitis B virus enhancer region[J]. Molecular and Cellular Biology, 1989, 9(7): 2787-2797. (  0) 0) |
| [6] |
Siegrist C A, Durand B, Emery P, et al. RFX1 is identical to enhancer factor C and functions as a transactivator of the hepatitis B virus enhancer[J]. Molecular and Cellular Biology, 1993, 13(10): 6375-6384. (  0) 0) |
| [7] |
Reith W, Ucla C, Barras E, et al. Rfx1, a transactivator of hepatitis-B virus enhancer-Ⅰ, belongs to a novel family of homodimeric and heterodimeric DNA-binding proteins[J]. Molecular and Cellular Biology, 1994, 14(2): 1230-1244. (  0) 0) |
| [8] |
Pugliatti L, Derre J, Berger R, et al. The genes for MHC classⅡ regulatory factors RFX1 and RFX2 are located on the short arm of chromosome 19[J]. Genomics, 1992, 13(4): 1307-1310. DOI:10.1016/0888-7543(92)90052-T (  0) 0) |
| [9] |
Dotzlaw H, Alkhalaf M, Murphy L C. Characterization of estrogen receptor variant mRNAs from human breast cancers[J]. Molecular Endocrinology, 1992, 6(5): 773-785. (  0) 0) |
| [10] |
Morotomi-Yano K, Yano K I, Saito H, et al. Human regulatory factor X4 (RFX4) is a testis-specific dimeric DNA-binding protein that cooperates with other human RFX members[J]. Journal of Biological Chemistry, 2002, 277(1): 836. DOI:10.1074/jbc.M108638200 (  0) 0) |
| [11] |
Steimle V, Durand B, Barras E, et al. A novel DNA-binding regulatory factor is mutated in primary MHC classⅡ deficiency (bare lymphocyte syndrome)[J]. Genes & Development, 1995, 9(9): 1021. (  0) 0) |
| [12] |
Chu J, Lucie S, Syed A, et al. Identification and characterization of novel human tissue-specific RFX transcription factors[J]. BMC Evolutionary Biology, 2008, 8(1): 226. DOI:10.1186/1471-2148-8-226 (  0) 0) |
| [13] |
Smith S B, Qu H Q, Taleb N, et al. Rfx6 directs islet formation and insulin production in mice and humans[J]. Nature, 2011, 463(5): 775-780. (  0) 0) |
| [14] |
Soyer J, Flasse L, Raffelsberger W, et al. Rfx6 is an Ngn3-dependent winged helix transcription factor required for pancreatic islet cell development[J]. Development, 2010, 137(2): 203-212. DOI:10.1242/dev.041673 (  0) 0) |
| [15] |
Smith S B, Qu H Q, Taleb N, et al. Rfx6 directs islet formation and insulin production in mice and humans[J]. Nature, 2010, 463(7282): 775-780. DOI:10.1038/nature08748 (  0) 0) |
| [16] |
Castro W, Chelbi S T, Niogret C, et al. The transcription factor Rfx7 limits metabolism of NK cells and promotes their maintenance and immunity[J]. Nature Immunology, 2018, 19(8): 809. DOI:10.1038/s41590-018-0144-9 (  0) 0) |
| [17] |
Sugiaman-Trapman D, Vitezic M, Jouhilahti E M, et al. Characterization of the human RFX transcription factor family by regulatory and target gene analysis[J]. BMC Genomics, 2018, 19(1): 181. DOI:10.1186/s12864-018-4564-6 (  0) 0) |
| [18] |
Piasecki B P, Burghoorn J, Swoboda P. Regulatory Factor X (RFX)-mediated transcriptional rewiring of ciliary genes in animals[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(29): 12969-12974. DOI:10.1073/pnas.0914241107 (  0) 0) |
| [19] |
Durand B, Vandaele C, Spencer D, et al. Cloning and characterization of dRFX, the Drosophila member of the RFX family of transcription factors[J]. Gene, 2000, 246(1-2): 285-293. DOI:10.1016/S0378-1119(00)00093-7 (  0) 0) |
| [20] |
Reith W, Herrero-Sanchez C, Kobr M, et al. MHC classⅡ regulatory factor RFX has a novel DNA-binding domain and a functionally independent dimerization domain[J]. Genes & Development, 1990, 4(9): 1528-1540. (  0) 0) |
| [21] |
Chen L, Smith L, Johnson M R, et al. activation of protein kinase c induces nuclear translocation of RFX1 and down-regulates c-myc via an intron 1 X box in undifferentiated leukemia hl-60 cells[J]. Journal of Biological Chemistry, 2018, 275(41): 32227-32233. (  0) 0) |
| [22] |
Iwama A, Jing P, Pu Z, et al. Dimeric RFX proteins contribute to the activity and lineage specificity of the interleukin-5 receptor alpha promoter through activation and repression domains[J]. Molecular and Cellular Biology, 1999, 19(6): 3940-3950. DOI:10.1128/MCB.19.6.3940 (  0) 0) |
| [23] |
Sengupta P K, Ehrlich M, Smith B D. A methylation-responsive MDBP/RFX site is in the first exon of the collagen α2(Ⅰ) promoter[J]. Journal of Biological Chemistry, 1999, 274(51): 36649. DOI:10.1074/jbc.274.51.36649 (  0) 0) |
| [24] |
Zhang X Y, Jabrane-Ferrat N, Asiedu C K, et al. The major histocompatibility complex classⅡ promoter-binding protein RFX (NF-X) is a methylated DNA-binding protein[J]. Molecular & Cellular Biology, 1993, 13(11): 6810. (  0) 0) |
| [25] |
Chung M I, Peyrot S M, LeBoeuf S, et al. RFX2 is broadly required for ciliogenesis during vertebrate development[J]. Developmental Biology, 2012, 363(1): 155-165. DOI:10.1016/j.ydbio.2011.12.029 (  0) 0) |
| [26] |
Bisgrove B W, Makova S, Yost H J, et al. RFX2 is essential in the ciliated organ of asymmetry and an RFX2 transgene identifies a population of ciliated cells sufficient for fluid flow[J]. Developmental Biology, 2012, 363(1): 166-178. DOI:10.1016/j.ydbio.2011.12.030 (  0) 0) |
| [27] |
Sedykh I, Keller A N, Yoon B, et al. Zebrafish Rfx4 controls dorsal and ventral midline formation in the neural tube[J]. Developmental Dynamics, 2018, 247(4): 650-659. DOI:10.1002/dvdy.24613 (  0) 0) |
| [28] |
曹木青, 潘俊敏. 纤毛及纤毛相关疾病研究进展[J]. 中国细胞生物学学报, 2012, 34(9): 849-856. Cao M Q, Pan J M. Cilia and ciliopathies[J]. Chinese Journal of Cell Biology, 2012, 34(9): 849-856. (  0) 0) |
| [29] |
Swoboda P, Adler H T, Thomas J H. The RFX-type transcription factor DAF-19 regulates sensory neuron cilium formation in C. elegans[J]. Molecular Cell, 2000, 5(3): 411-421. DOI:10.1016/S1097-2765(00)80436-0 (  0) 0) |
| [30] |
Dubruille R, Laurenon A, Vandaele C, et al. Drosophila regulatory factor X is necessary for ciliated sensory neuron differentiation[J]. Development, 2003, 129(23): 5487-5498. (  0) 0) |
| [31] |
Baas D, Meiniel A, Benadiba C, et al. A deficiency in RFX3 causes hydrocephalus associated with abnormal differentiation of ependymal cells[J]. European Journal of Neuroscience, 2010, 24(4). (  0) 0) |
| [32] |
El Zein L, Ait-Lounis A, Morle L, et al. RFX3 governs growth and beating efficiency of motile cilia in mouse and controls the expression of genes involved in human ciliopathies[J]. Journal of Cell Science, 2009, 122(Pt17): 3180-3189. (  0) 0) |
| [33] |
Ait-Lounis A, Baas D, Barras E, et al. Novel function of the ciliogenic transcription factor RFX3 in development of the endocrine pancreas[J]. Diabetes, 2007, 56(4): 950-959. DOI:10.2337/db06-1187 (  0) 0) |
| [34] |
Bonnafe E, Touka M, AitLounis A, et al. The transcription factor RFX3 directs nodal cilium development and left-right asymmetry specification[J]. Molecular and Cell Biology, 2004, 24(10): 4417-4427. DOI:10.1128/MCB.24.10.4417-4427.2004 (  0) 0) |
| [35] |
Essner J J, Vogan K J, Wagner M K, et al. Conserved function for embryonic nodal cilia[J]. Nature, 2002, 418(6893): 37-38. DOI:10.1038/418037a (  0) 0) |
| [36] |
Yu X W, Ng C P, Habacher H, et al. Foxj1 transcription factors are master regulators of the motile ciliogenic program[J]. Nature Genetics, 2008, 40(12): 1445-1453. DOI:10.1038/ng.263 (  0) 0) |
| [37] |
Hellman N E, Liu Y, Merkel E, et al. The zebrafish foxj1a transcription factor regulates cilia function in response to injury and epithelial stretch[J]. Proceedings of the National Academy of Sciences, 2010, 107(43): 18499-18504. DOI:10.1073/pnas.1005998107 (  0) 0) |
| [38] |
Ashique A M, Choe Y, Karlen M, et al. The Rfx4 transcription factor modulates shh signaling by regional control of ciliogenesis[J]. Science Signaling, 2009, 2(95): 70. (  0) 0) |
| [39] |
Manojlovic Z, Earwood R, Kato A, et al. RFX7 is required for the formation of cilia in the neural tube[J]. Mechanisms of Development, 2014, 132: 28-37. DOI:10.1016/j.mod.2014.02.001 (  0) 0) |
| [40] |
Purvis T L, Hearn T, Spalluto C, et al. Transcriptional regulation of the alström syndrome gene ALMS 1 by members of the RFX family and Sp1[J]. Gene, 2010, 460(1-2): 20-29. DOI:10.1016/j.gene.2010.03.015 (  0) 0) |
| [41] |
Neto F T L, Bach P V, Najari B B, et al. Spermatogenesis in humans and its affecting factors[J]. Seminars in Cell & Developmental Biology, 2016, 59: 10-26. (  0) 0) |
| [42] |
Kistler W S, Horvath G C, Dasgupta A, et al. Differential expression of Rfx1—4 during mouse spermatogenesis[J]. Gene Expression Patterns, 2009, 9(7): 515-519. DOI:10.1016/j.gep.2009.07.004 (  0) 0) |
| [43] |
Bellora N, Farré D, Albà M. Positional bias of general and tissue-specific regulatory motifs in mouse gene promoters[J]. BMC Genomics, 2007, 8(1): 459. DOI:10.1186/1471-2164-8-459 (  0) 0) |
| [44] |
Horvath G C, Kistler W S, Kistler M K. RFX2 is a potential transcriptional regulatory factor for histone H1t and other genes expressed during the meiotic phase of spermatogenesis[J]. Biology Reprodction, 2004, 71(5): 1551-1559. DOI:10.1095/biolreprod.104.032268 (  0) 0) |
| [45] |
Wolfe S A, van Wert J, Grimes S R. Transcription factor RFX2 is abundant in rat testis and enriched in nuclei of primary spermatocytes where it appears to be required for transcription of the testis-specific histone H1t gene[J]. Journal of Cellular Biochemistry, 2006, 99(3): 735-746. DOI:10.1002/jcb.20959 (  0) 0) |
| [46] |
Grimes S, Weisz-Carrington P, Daum H, et al. A rat histone H4 gene closely associated with the testis-specific H1t gene[J]. Experimental Cell Research, 1987, 173(2): 534-545. DOI:10.1016/0014-4827(87)90293-X (  0) 0) |
| [47] |
Horvath G C, Kistler M K, Kistler W S. RFX2 is a candidate downstream amplifier of A-MYB regulation in mouse spermatogenesis[J]. BMC Developmental Biology, 2009, 9(1): 63. DOI:10.1186/1471-213X-9-63 (  0) 0) |
| [48] |
Wu Y, Hu X, Li Z, et al. Transcription factor RFX2 is a key regulator of mouse spermiogenesis[J]. Scientific Reports, 2015, 6: 20435. (  0) 0) |
| [49] |
Kistler W S, Baas D, Lemeille S, et al. RFX2 Is a Major Transcriptional Regulator of Spermiogenesis[J]. PLoS Genetics, 2015, 11(7): e1005368. DOI:10.1371/journal.pgen.1005368 (  0) 0) |
| [50] |
Jiang J, Zhang N, Shiba H, et al. Spermatogenesis associated 4 promotes sertoli cell proliferation modulated negatively by regulatory factor X1[J]. PLoS One, 2013, 8(10): e75933. DOI:10.1371/journal.pone.0075933 (  0) 0) |
| [51] |
Wang B, Qi T, Chen S Q, et al. RFX1 maintains testis cord integrity by regulating the expression of Itga6 in male mouse embryos[J]. Molecular Reproduction & Development, 2016, 83(7): 606-6142. (  0) 0) |
| [52] |
Wolfe S A, Vanwert J M, Grimes S R. Transcription factor RFX4 binding to the testis-specific histone H1t promoter in spermatocytes may be important for regulation of H1t gene transcription during spermatogenesis[J]. Journal of Cellular Biochemistry, 2010, 105(1): 61-69. (  0) 0) |
| [53] |
Xie Y, Moussaif M, Choi S, et al. RFX transcription factor DAF-19 regulates 5-HT and innate immune responses to pathogenic bacteria in Caenorhabditis elegans[J]. PLoS Genet, 2013, 9(3): e1003324. DOI:10.1371/journal.pgen.1003324 (  0) 0) |
| [54] |
Villard J, Mach B, Reith W. MHC classⅡ deficiency: Definition of a new complementation group[J]. Immunobiology, 1997, 198(1-3): 264-272. DOI:10.1016/S0171-2985(97)80046-0 (  0) 0) |
| [55] |
Knight, Julian, C, et al. Genomic mapping of the MHC transactivator CIITA using an integrated ChIP-seq and genetical genomics[J]. Genome Biology, 2014, 15: 494. DOI:10.1186/s13059-014-0494-z (  0) 0) |
| [56] |
Nekrep N, Fontes J D, Geyer M, et al. When the lymphocyte loses its clothes[J]. Immunity, 2003, 18(4): 453-457. DOI:10.1016/S1074-7613(03)00086-4 (  0) 0) |
| [57] |
Devaiah B N, Singer D S. CIITA and its dual roles in MHC gene transcription[J]. Frontiers in Immunology, 2013, 4(476): 476. (  0) 0) |
| [58] |
Beaulieu Y B, Machado J, Ethier S, et al. Degradation, promoter recruitment and transactivation mediated by the extreme N-terminus of MHC classⅡ transactivator CIITA isoformⅢ[J]. PLoS One, 2016, 11(2): e0148753. DOI:10.1371/journal.pone.0148753 (  0) 0) |
| [59] |
Gao J, Xu C. Structural basis for the recognition of RFX7 by ANKRA2 and RFXANK[J]. Biochemical & Biophysical Research Communications, 2020, 523(1): 263-266. (  0) 0) |
| [60] |
Zhao M, Tan Y, Peng Q, et al. IL-6/STAT3 pathway induced deficiency of RFX1 contributes to Th17-dependent autoimmune diseases via epigenetic regulation[J]. Nature Communications, 2018, 9(1): 583. DOI:10.1038/s41467-018-02890-0 (  0) 0) |
| [61] |
Bugeja H E, Hynes M J, Andrianopoulos A. The RFX protein RfxA is an essential regulator of growth and morphogenesis in Penicillium marneffei[J]. Eukaryotic Cell, 2010, 9(4): 578-591. DOI:10.1128/EC.00226-09 (  0) 0) |
| [62] |
Schmitt E K. The fungal CPCR1 protein, which binds specifically to beta-lactam biosynthesis genes, is related to human regulatory factor X transcription factors[J]. Journal of Biological Chemistry, 2000, 275(13): 9348-9357. DOI:10.1074/jbc.275.13.9348 (  0) 0) |
| [63] |
Wu S Y, Mcleod M. The sak1 + gene of Schizosaccharomyces pombe encodes an RFX family DNA-binding protein that positively regulates cyclic AMP-dependent protein kinase-mediated exit from the mitotic cell cycle[J]. Molecular & Cellular Biology, 1995, 15(3): 1479-1488. (  0) 0) |
| [64] |
Huang M, Zheng Z, Elledge S J. The DNA replication and damage checkpoint p athways induce transcription by inhibition of the Crt 1 repressor[J]. Cell, 1998, 94(5): 595-605. DOI:10.1016/S0092-8674(00)81601-3 (  0) 0) |
| [65] |
Labrie C, Lee B H, Mathews M B. Transcription factors RFX1/EF-C and ATF-1 associate with the adenovirus E1A-responsive element of the human proliferating cell nuclear antigen promoter[J]. Nucleic Acids Research, 1995, 23(18): 3732-3741. DOI:10.1093/nar/23.18.3732 (  0) 0) |
| [66] |
Suski J M, Braun M, Strmiska V, et al. Targeting cell-cycle machinery in cancer[J]. Cancer Cell, 2021, 39(6): 759-778. DOI:10.1016/j.ccell.2021.03.010 (  0) 0) |
| [67] |
赵杨静, 谢兴旺, 王荟, 等. 调节因子X5及其复合物的研究进展[J]. 临床检验杂志, 2017, 35(5): 356-359. Zhao Y J, Xie X W, Wang H, et al. Chinese Journal of Clinical Laboratory Science, 2017, 35(5): 356-359. (  0) 0) |
| [68] |
Zhao Y J, Xie X W, Liao W J, et al. The transcription factor RFX5 is a transcriptional activator of the TPP1 gene in hepatocellular carcinoma[J]. Oncology Reports, 2017, 37(1): 289-296. DOI:10.3892/or.2016.5240 (  0) 0) |
| [69] |
Epstein F H, Blobe G C, Schiemann W P, et al. Role of transforming growth factor beta in human disease[J]. New England Journal of Medicine, 2000, 342(18): 1350-1358. DOI:10.1056/NEJM200005043421807 (  0) 0) |
| [70] |
Feng C, Zuo Z. Regulatory factor X1-induced down-regulation of transforming growth factor β2 transcription in human neuroblastoma cells[J]. Journal of Biological Chemistry, 2012, 287(27): 22730-22739. DOI:10.1074/jbc.M111.338590 (  0) 0) |
| [71] |
Su J C, Chiang H C, Tseng P H, et al. RFX-1-dependent activation of SHP-1 inhibits STAT3 signaling in hepatocellular carcinoma cells[J]. Carcinogenesis, 2014, 35(12): 2807-2814. DOI:10.1093/carcin/bgu210 (  0) 0) |
| [72] |
Jeong H Y, Kim H J, Cheol-Eun K, et al. High expression of RFX4 is associated with tumor progression and poor prognosis in patients with glioblastoma[J]. International Journal of Neuroscience, 2020, 131(10): 1-11. (  0) 0) |
| [73] |
Coronel L, Riege K, Schwab K, et al. Transcription factor RFX7 governs a tumor suppressor network in response to p53 and stress[J]. Nucleic Acids Research, 2021, 49(13): 7437-7456. DOI:10.1093/nar/gkab575 (  0) 0) |
| [74] |
Baas D, Meiniel A, Benadiba C, et al. A deficiency in RFX3 causes hydrocephalus associated with abnormal differentiation of ependymal cells[J]. European Journal of Neuroscience, 2006, 24(4): 1020-1030. DOI:10.1111/j.1460-9568.2006.05002.x (  0) 0) |
| [75] |
Lemeille S, Paschaki M, Baas D, et al. Interplay of RFX transcription factors 1, 2 and 3 in motile ciliogenesis[J]. Nucleic Acids Research, 2020, 48(16): 9019-9036. DOI:10.1093/nar/gkaa625 (  0) 0) |
| [76] |
蔡昆争, 骆世明, 段舜山. 生命系统的功能冗余[C]//第12届全国农业生态学研讨会. 广州: 中国生态学会, 广东省生态学会, 2005: 381-384. Cai K Z, Luo S M, Duan S S. Functional redundancy of living systems[C]//The 12th National Conference on Agricultural Ecology. Guangzhou: Chinese Ecological Society, Guangdong Ecological Society, 2005: 381-384. (  0) 0) |
| [77] |
Lu J, Cheng C, Cheng Z C, et al. The dual role of RFX6 in directing beta cell development and insulin production[J]. Journal of Molecular Endocrinology, 2021, 66(2): 129-139. DOI:10.1530/JME-20-0119 (  0) 0) |
| [78] |
Elkon R, Milon B, Morrison L, et al. RFX transcription factors are essential for hearing in mice[J]. Nature Communication, 2015, 6: 8549. DOI:10.1038/ncomms9549 (  0) 0) |
 2023, Vol. 53
2023, Vol. 53

