2. 青岛海洋科学与技术国家实验室, 海洋渔业科学与食物产出过程功能实验室, 山东 青岛 266200
长牡蛎(Crassostrea gigas)也称太平洋牡蛎,自然分布于西北太平洋海域,具有生长速度快、养殖成本低、抗逆性高、适应性强等特点,是世界性海产贝类养殖种类之一。因其巨大的养殖规模和经济价值,目前关于其抗病、产量等重要经济性状的良种选育研究已相继展开[1-3]。
壳色是海产经济贝类的一个重要经济性状,受消费者喜爱的壳色可以显著提高其商品价值。贝类壳色的多态性在海产经济贝类中普遍存在[4-6]。长牡蛎壳色主要受遗传影响且遗传力较高,经人工选育可实现对壳色的纯化[7-8]。采用家系选育和群体选育的混合选育技术,我们培育出左右壳均为黑色、遗传稳定的长牡蛎壳色新品系。该新选育品系不仅具靓丽的外观,还具有生长快、抗逆性强等优点,具有巨大的产业推广价值。长牡蛎壳黑选育品系的产业化应用对于促进牡蛎养殖业的良种化具有重要的意义。
幼虫培育是苗种规模化繁育工作的核心,也是成贝健康生长的重要基础。双壳贝类受精卵孵化后进入营浮游生活的幼虫期,浮游期贝类幼虫的发育由早期依赖自身卵黄和外源饵料的混合型营养迅速变为单纯依赖饵料的外源性营养[9]。可见,投饵量是影响贝类幼虫生长存活及后期附着变态的重要因素[10-13]。除投饵量外,培育密度对贝类幼虫生长和存活也有显著影响[14-17]。不同的贝类幼虫培育密度,其投饵量也不尽相同。Liu等[18]研究表明培育密度和投饵量对幼虫生长存活有显著互作效应。Marshall等[19]研究也表明培育密度和饵料量适量增加对幼虫生长存活存在协同作用,但过高的培育密度会与投饵量产生拮抗作用,不利于幼虫生长。因此,确定适宜的培育密度和投饵量是贝类苗种规模化繁育的关键技术问题。
本研究以长牡蛎野生群体幼虫为对照组,研究了不同培育密度和投饵量对长牡蛎壳黑选育品系幼虫生长、存活及附着变态的影响,旨在阐明长牡蛎壳黑选育品系与野生群体幼虫生长与存活性状的差异,确定适宜的培育密度和投饵量,为长牡蛎壳黑选育品系产业化应用提供基础数据。
1 材料与方法 1.1 亲贝采集2017年5月从山东荣成海域养殖的第7代长牡蛎壳黑选育群体中,挑选左右壳均为黑色个体作为亲贝,并采集同海区野生长牡蛎亲贝作为对照组,进行室内人工促熟。亲贝促熟期间,海水温度为19 ~ 22 ℃,盐度为33。每2天100%换水1次,每日投喂新月菱形藻(Nschia closterium)和球等鞭金藻(Isochrysis galbana)1:1的混合饵料4次。
1.2 幼虫孵化长牡蛎壳黑品系和野生群体的亲贝性腺发育成熟后,通过解剖法辨别雌雄后,分别进行人工授精。受精卵孵化期间进行持续微充气,水温22 ℃,孵化密度为20~30个/mL。受精卵经过24h发育为D形幼虫。
1.3 实验设计实验设置3个幼虫培育密度梯度(3、7和15个/mL)和3个初始投饵量梯度(0.4、0.8和1.6×103cells·ind-1·d-1)的交互组合共9个实验组,每组均设3个重复组。取长牡蛎壳黑选育品系和野生群体2日龄D形幼虫,其壳高分别为(69.80±8.34) μm和(67.40±8.04) μm,随机分组,按上述梯度组合分别转移到80 L水体的聚乙烯塑料桶内充气培养,日常管理与亲贝促熟期间一致。实验期间,投喂球等鞭金藻,每次投喂前,采用血球计数板测定饵料浓度,定量投喂。
实验期间,随日龄增加幼虫规格逐渐增大,摄食量增加。在维持投饵量梯度比相同的基础上,逐渐增加投饵量(见表 1)。
|
|
表 1 实验期间幼虫每日投饵量 Table 1 Daily feed ration for larvae reared at different algal feed ration during the experimental period |
实验第5、10、15和20天从各实验组随机取50个幼虫,卢戈氏液固定,在显微镜下测定幼虫壳高。实验结束时,测量各实验组幼虫平均壳高并与初始壳高相比较,计算各实验组生长速率;测定各实验组幼虫密度,计算各实验组存活率。当眼点幼虫比例>50%时,投放附着基。投放7 d后,测定各实验组幼虫附着率[11]。
1.4 数据分析实验数据均采用平均值±标准差(Mean± SD)表示,采用SPSS19.0进行数据分析,以培育密度和投饵量为影响因素,进行双因素方差分析(two-way ANOVA)。当培育密度和投饵量之间存在显著交互作用时,对数据进行一次单因素方差分析(One-way ANOVA),并采用Tukey’s检验进行多重比较分析各实验组间的显著性。长牡蛎壳黑选育品系与野生群体对照组之间采用独立样本t检验,显著性水平均设为P < 0.05。
2 结果 2.1 培育密度和投饵量对长牡蛎幼虫生长的影响培育密度和投饵量对长牡蛎幼虫壳高生长有显著影响(P < 0.05)(见表 2)。实验第5天,在培育密度15个/mL实验组长牡蛎壳黑品系D形幼虫壳高显著高于培育密度3个/mL实验组(P < 0.05),并在培育密度15个/mL、投饵量1.6×103cells·ind-1·d-1实验组,壳黑品系D形幼虫壳高达到最大值为(107.70±3.97)μm。实验期间,随日龄增加幼虫规格逐渐增大,最适培育条件逐步发生变化。第15和20天,长牡蛎壳黑品系与野生群体的壳顶幼虫和眼点幼虫壳高均在培育密度7个/mL,投饵量1.6×103cells·ind-1d-1实验组达到最大值,显著高于其它实验组(P < 0.05)。此外,在培育密度15个/mL实验组壳黑选育品系各时期幼虫壳高始终高于野生群体幼虫。
|
|
表 2 不同培育密度和投饵量对壳黑选育品系和野生群体幼虫壳高生长的影响 Table 2 Growth of black shell color strain and wild population of Crassostrea gigas larvae reared under different combinations of stocking density and feed ration |
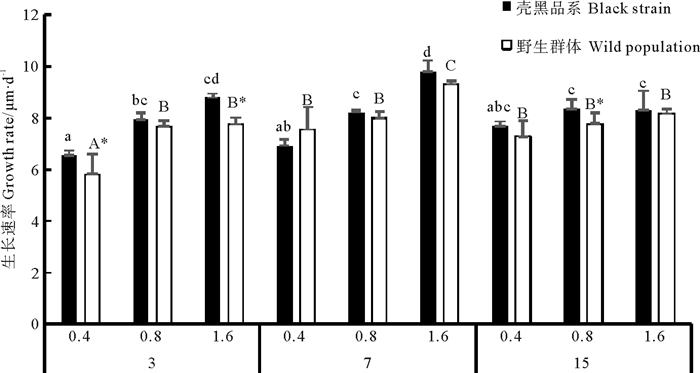
培育密度对长牡蛎野生群体幼虫生长速率有显著影响(P < 0.05),对长牡蛎壳黑品系幼虫影响不显著(P> 0.05),培育密度和投饵量对长牡蛎幼虫生长速率存在显著交互作用(P < 0.05)(见表 3)。在培育密度3和7个/mL实验组,随投饵量增加长牡蛎幼虫生长速率显著增大(P < 0.05),并在培育密度7个/mL、投饵量1.6×103cells·ind-1·d-1实验组,长牡蛎壳黑品系和野生群体幼虫生长速率均达到最大值,分别为(9.80± 0.62)μm·d-1和(9.30± 0.75)μm·d-1(见图 1)。而在培育密度15个/mL实验组,投饵量增加对幼虫生长速率无显著影响(P> 0.05),且各饵料投喂组生长速率均显著低于培育密度7个/mL、投饵量1.6×103 cells·ind-1·d-1实验组(P> 0.05)。除培育密度7个/mL、投饵量0.4×103 cells·ind-1·d-1实验组外,壳黑品系幼虫生长速率均高于野生群体幼虫。
|
|
表 3 培育密度和投饵量对长牡蛎壳黑品系与野生群体幼虫生长速率、存活率及附着率影响的方差分析 Table 3 Two-way ANOVA comparing the effect of stocking density and algal feed ration on growthsurvival and settlement rate of black shell color strain and wild population of C. gigas larvae |
|
(3、7和15表示培育密度为3、7和15个/mL,0.4、0.8和1.6表示初始投饵量为0.4×103、0.8×103和1.6×103cells·ind-1·d-1, *表示差异显著(P < 0.05)。下同。Stocking density of 3, 7 and 15 inds·mL-1 be represented as 3, 7 and 15. Initial feed ration of 0.4×103, 0.8×103 and 1.6×103cells·ind-1·d-1 be represented as 0.4, 0.8 and 1.6. Significant differences are denoted by asterisks (P < 0.05). The same as below.) 图 1 不同培育密度和投饵量对壳黑品系和野生群体幼虫生长速率的影响 Fig. 1 Growth rates of black shell color strain and wild population of C. gigas larvae at different larval stocking density and algal feed ration |
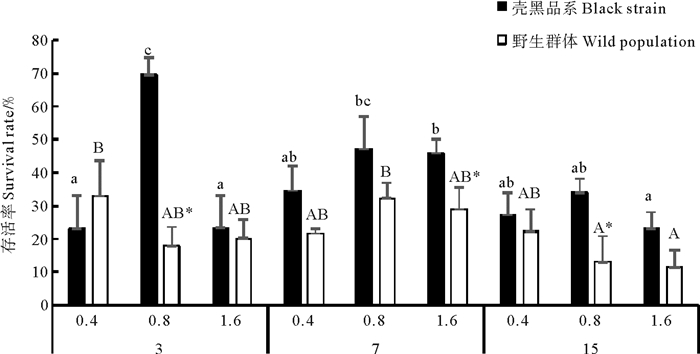
培育密度和投饵量对长牡蛎幼虫存活率存在显著交互作用(P < 0.05),投饵量对长牡蛎壳黑品系幼虫存活率影响显著(P < 0.05),对长牡蛎野生群体幼虫存活率无显著影响(P> 0.05)(见表 3)。在培育密度3和15个/mL实验组,随投饵量增加壳黑品系幼虫存活率先升高后下降,野生群体幼虫各饵料投喂组间差异不显著(P> 0.05)(见图 2)。在培育密度3个/mL,投饵量0.8×103 cells·ind-1·d-1实验组,壳黑品系幼虫存活率为69.58%,显著高于其它实验组(P < 0.05),最终幼虫存活数量为2.09个/mL。在培育密度15个/mL、投饵量1.6×103 cells·ind-1·d-1实验组,长牡蛎壳黑选育品系和野生群体幼虫存活率最低,分别为23.01%和11.43%,最终幼虫存活数量分别为3.45和1.71个/mL。在培育密度7个/mL实验组,长牡蛎幼虫各饵料投喂组间差异不显著(P> 0.05)。在培育密度7个/mL、投饵量1.6×103cells·ind-1·d-1实验组壳黑选育品系幼虫存活率为45.83%,最终幼虫存活数量为3.21个/mL;两群体比较中,除培育密度3个/mL、投饵量0.4×103 cells·ind-1·d-1实验组外,壳黑选育品系幼虫存活率均高于野生群体幼虫。
|
图 2 不同培育密度和投饵量对壳黑品系和野生群体幼虫存活率的影响 Fig. 2 Survival rates of black shell color strain and wild population of C. gigaslarvae at different larval stocking density and algal feed ration |
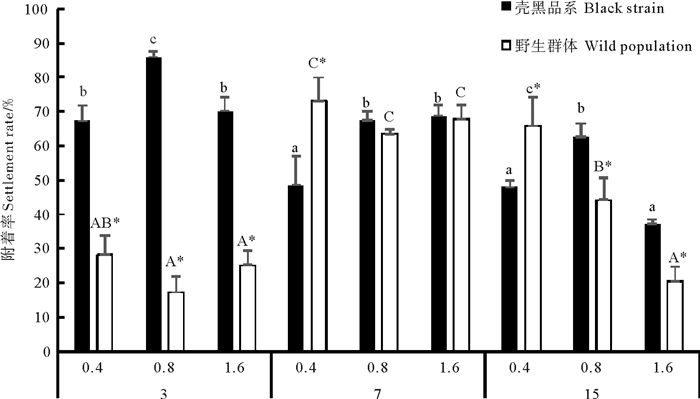
培育密度和投饵量对长牡蛎幼虫附着率有显著交互作用(P < 0.05)(见表 3)。在培育密度3个/mL实验组,随投饵量增加长牡蛎壳黑品系幼虫附着率先升高后下降,并在投饵量0.8×103 cells·ind-1·d-1实验组长牡蛎壳黑品系幼虫附着率显著高于其它实验组(P < 0.05);在培育密度7个/mL实验组,随投饵量增加长牡蛎壳黑品系幼虫附着率增大,长牡蛎野生群体幼虫附着率各饵料实验组间差异不显著(P> 0.05)。随培育密度增加,长牡蛎壳黑品系幼虫附着率逐步降低。在培育密度15个/mL,投饵量1.6×103 cells·ind-1·d-1实验组,长牡蛎壳黑品系幼虫附着率最低。且培育密度15个/mL实验组,随投饵量增加长牡蛎野生群体幼虫附着率显著降低(P < 0.05)(见图 3)。除培育密度7个/mL、投饵量0.4×103 cells·ind-1·d-1和培育密度15个/mL、投饵量0.4×103 cells·ind-1·d-1实验组外,长牡蛎壳黑品系幼虫附着率均高于野生群体。
|
图 3 不同培育密度和投饵量对壳黑品系和野生群体幼虫附着率的影响 Fig. 3 Settlement rates of black shell color strain and wild population of C. gigaslarvae at different larval stocking density and algal feed ration |
在适宜范围内,增加培育密度和投饵量会增大贝类幼虫与饵料的相碰机率,提高幼虫的摄食效率,降低摄食行为所造成的能量消耗,对贝类幼虫的生长有促进作用[18-19]。本研究结果表明,在培育密度3和7个/mL实验组,随投饵量增加长牡蛎幼虫生长速率显著增大,并在培育密度7个/mL、投饵量1.6×103 cells·ind-1·d-1实验组长牡蛎幼虫生长速率最高。而在培育密度15个/mL实验组,投饵量增加对长牡蛎幼虫生长速率没有显著影响。这表明高培育密度和饵料量之间存在拮抗作用,对长牡蛎幼虫生长速率产生影响。相似的研究结果在珠母贝(Pinctada margaritifera)[15]、鸟蛤(Clinocardium nuttallii)[18]、象拔蚌(Panopea generosa)[19]也有报道。这种拮抗作用可能与贝类幼虫个体间竞争加剧和过饱食有关。Liu等[18]研究表明在过高的培育密度条件下,贝类幼虫个体间竞争加剧,其生长受到抑制。Ricovilla等[9]研究表明随投饵量增大,贝类幼虫摄食量逐步增加,生长速率增大。但幼虫的摄食量存在阈值,超出阈值会使幼虫过饱食。过饱食状态下幼虫会因消化器官负载而产生拒绝摄食的现象[13, 20],并产生过多假粪,增加机体能量支出使生长速率降低。而在过高的培育密度条件下,增加投饵量,贝类幼虫会因摄食效率的提高更容易达到过饱食状态,不利于生长发育[19]。实验期间,不同发育阶段长牡蛎壳黑选育品系幼虫的最适培育条件存在差异。从D形幼虫到壳顶幼虫及眼点幼虫,其最适培育密度组由15个/mL降低至7个/mL。其原因可能是在培育初期,壳黑品系幼虫规格较小,较高的培育密度环境下,有利于提高幼虫摄食效率。而随着日龄增加,幼虫规格增大,个体间竞争加剧,高培育密度环境不利于幼虫生长。Doroudi等[15]也得出相似结果,认为在珠母贝幼虫在8日龄时,最适培育密度3个/mL,而在20日龄时,培育密度应小于2个/mL。因此,本研究表明长牡蛎壳黑品系幼虫培育期间,初期较高的培育密度和投饵量有利其快速生长发育,当壳黑品系幼虫壳高超过(142.00±1.00) μm时,降低培育密度有利于幼虫生长和存活。
存活率是评价贝类幼虫培育环境的指标,是幼虫对不良环境耐受能力的体现[15]。本实验结果表明过高培育密度对长牡蛎壳黑品系和野生群体幼虫存活率均产生负面影响,两群体幼虫均在培育密度15个/mL、投饵量1.6×103 cells·ind-1·d-1实验组存活率最低,分别为23.01%和11.43%。相似的研究结论在珠母贝[15]、象拔蚌[19]、毛蚶(Scapharca subcrenata) [21]也有报道。培育密度过高会使水体中溶解氧含量降低,氨氮积累过多。在氨氮浓度较高的环境中,机体生理功能会受到负面影响,不利于贝类幼虫生长存活[22]。同时,粪便累积败坏水质,加速水中致病原生动物及细菌的滋生,导致幼虫存活率下降[15]。投饵量适量增加可为贝类幼虫提供充足的营养提高存活率。而过高的投饵量会使养殖水体中硝酸盐含量上升,导致幼虫的死亡率升高[23-24]。
附着变态是贝类由浮游幼虫向稚贝转变的重要发育阶段。当培育环境不适宜时,贝类幼虫会延长附着变态时间,最终导致大量死亡[25]。因此,附着率的高低是苗种培育工作成功的重要因素。本实验结果表明,投饵量适量增加有利于贝类幼虫附着,但过高的投饵量会使幼虫附着率降低。且随培育密度增大长牡蛎壳黑品系幼虫附着率逐步降低。类似的结果在其它双壳贝类也有报道。Robert等[26]研究发现食用牡蛎(Ostrea edulis)随饵料量增加其附着率先升高后降低。Liu等[27]对文蛤(Meretrix meretrix)研究表明培育密度增加会导致文蛤幼虫附着变态时间的延长。高霄龙等[21]对毛蚶研究也表明随培育密度增加,不仅毛蚶幼虫附着变态时间被延长,且变态率也逐渐降低。出现此现象的原因可能是适量增加投饵量可补充贝类幼虫附着变态所需的能量,投饵过多会增加残饵导致水质恶化,不利于幼虫附着。高培育密度环境下,因贝类幼虫生长缓慢其附着变态需要更多时间。且存活率实验结果表明在高培育密度环境下长牡蛎幼虫的死亡率会上升,这会进一步降低长牡蛎幼虫附着率。
3.2 长牡蛎壳黑品系与野生群体幼虫的比较研究表明,不同壳色的同种贝类在生长和存活等性状上存在差异[28-30],本研究中也发现培育密度对长牡蛎野生群体幼虫生长速率影响显著,而对长牡蛎壳黑品系幼虫影响不显著。且实验期间,在培育密度15个/mL实验组长牡蛎壳黑品系幼虫壳高始终高于野生群体幼虫。此外,除投饵量0.4×103cells·ind-1·d-1实验组外,长牡蛎壳黑品系幼虫生长速率、存活率和附着率均高于野生群体幼虫。这些性状优势可能与累代的人工定向选择有关。人工选择育种对双壳贝类生长、存活和抗病等性状的改良已有诸多报道,国内外已成功培育出长牡蛎[31]、悉尼岩牡蛎[32]、马氏珠母贝(Pinctada fucata)[33]和虾夷扇贝(Patinopecten yessoensis)[34]等经济贝类的快速生长选育品系;长牡蛎抗疱疹病毒(OsHV-1)选育系[35]和悉尼岩牡蛎抗寄生虫病(QX)选育品系[36]等培育工作也取得了较好研究成果。
邓传敏等[37]研究发现长牡蛎壳金选育系的摄食率显著高于普通群体。Bayne等[38]对悉尼岩牡蛎研究中也发现其快速生长选育系摄食能力显著优于野生群体。这表明双壳贝类选育品系有更高摄食能力,充足的饵料摄食量是发挥其性状优势的前提。本研究也发现投饵量对长牡蛎壳黑品系幼虫存活率影响显著。且在0.4×103cells·ind-1·d-1投饵量组,长牡蛎壳黑选育品系幼虫生长速率、存活率与附着率出现低于野生群体幼虫现象,这表明长牡蛎壳黑选育品系幼虫对饵料量有更高的需求,而低饵料投喂量不能为其提供充足的营养需求会导致其生长速率、存活率和附着率降低。
4 结语本文研究了培育密度和投饵量及其协同作用对长牡蛎壳黑品系和野生群体幼虫的生长、存活及附着变态的影响。结果表明,D形幼虫时期长牡蛎壳黑选育品系最适培育条件为培育密度15个/mL、投饵量1.6×103cells·ind-1·d-1。但随着幼虫规格的增大,过高的培育密度和饵料量之间存在拮抗作用,并不利于幼虫生长发育。在壳顶幼虫和眼点幼虫时期,其最适培育条件为培育密度7个/mL、投饵量1.6×103cells·ind-1·d-1。相对于野生群体幼虫,壳黑选育品系幼虫有着更高的饵料需求,能适应更高的培育密度环境,其生长、存活和附着性状优于野生群体幼虫。
| [1] |
Dégremont L, Garcia C, Jr A S. Genetic improvement for disease resistance in oysters: A review[J]. Journal of Invertebrate Pathology, 2015, 131: 226-241. DOI:10.1016/j.jip.2015.05.010
(  0) 0) |
| [2] |
Langdon C, Evans F, Jacobson D, et al. Yields of cultured pacific oysters Crassostrea gigas thunberg improved after one generation of selection[J]. Aquaculture, 2003, 220(1): 227-244.
(  0) 0) |
| [3] |
Melo C M R D, Durland E, Langdon C. Improvements in desirable traits of the Pacific oyster, a result of five generations of selection on the West Coast, USA[J]. Aquaculture, 2016, 460: 105-115. DOI:10.1016/j.aquaculture.2016.04.017
(  0) 0) |
| [4] |
张跃环, 闫喜武, 张澎, 等. 贝类壳色多态的研究概况及展望[J]. 水产科学, 2008, 27(12): 680-683. Zhang Y H, Yan X W, Zhang P, et al. Research advances and prospects on shell color polymorphism of mollusks[J]. Fisheries Science, 2008, 27(12): 680-683. DOI:10.3969/j.issn.1003-1111.2008.12.020 (  0) 0) |
| [5] |
Innes D J, Haley L E. Inheritance of a shell-color polymorphism in the mussel[J]. The Journal of Heredity, 1977, 68(3): 203-204. DOI:10.1093/oxfordjournals.jhered.a108814
(  0) 0) |
| [6] |
Peignon J M, Gerard A, Naciri Y, et al. Analysis of shell color determination in the Manila clam Ruditapes philippinarum[J]. Aquatic Living Resources, 1995, 8(2): 181-189. DOI:10.1051/alr:1995015
(  0) 0) |
| [7] |
Evans S, Camara M D, Langdon C J. Heritability of shell pigmentation in the Pacific oyster, Crassostrea gigas[J]. Aquaculture, 2009, 286(3): 211-216.
(  0) 0) |
| [8] |
Ge J, Li Q, Yu H, et al. Mendelian inheritance of golden shell color in the Pacific oyster Crassostrea gigas[J]. Aquaculture, 2015, 441: 21-24. DOI:10.1016/j.aquaculture.2015.01.031
(  0) 0) |
| [9] |
Rico-Villa B, Pouvreau S, Robert R. Influence of food density and temperature on ingestion, growth and settlement of Pacific oyster larvae, Crassostrea gigas[J]. Aquaculture, 2009, 287(3): 395-401.
(  0) 0) |
| [10] |
Robert R, Vignier J, Petton B. Influence of feeding regime and temperature on development and settlement of oyster Ostrea edulis (Linnaeus, 1758) larvae[J]. Aquaculture Research, 2017, 48(9): 4756-4773. DOI:10.1111/are.2017.48.issue-9
(  0) 0) |
| [11] |
Pérez-Camacho A, Belras R, Albentosa M. Effects of algal food concentration and body size on the ingestion rates of Ruditapes decussatus (Bivalvia) veliger larvae[J]. Marine Ecology Progress, 1994, 115(1): 87-92.
(  0) 0) |
| [12] |
Beiras R, Camacho A P. Influence of food concentration on the physiological energetics and growth of Ostrea edulis larvae[J]. Marine Biology, 1994, 120(3): 427-435. DOI:10.1007/BF00680217
(  0) 0) |
| [13] |
Tang B, Liu B, Wang G, et al. Effects of various algal diets and starvation on larval growth and survival of Meretrix meretrix[J]. Aquaculture, 2006, 254(1-4): 526-533. DOI:10.1016/j.aquaculture.2005.11.012
(  0) 0) |
| [14] |
Yan X, Zhang G, Yang F. Effects of diet, stocking density, and environmental factors on growth, survival, and metamorphosis of Manila clam Ruditapes philippinarum larvae[J]. Aquaculture, 2006, 253(1): 350-358.
(  0) 0) |
| [15] |
Doroudi M S, Southgate P C. The influence of algal ration and larval density on growth and survival of blacklip pearl oyster Pinctada margaritifera(L.)larvae[J]. Aquaculture Research, 2000, 31(8-9): 621-626. DOI:10.1046/j.1365-2109.2000.00483.x
(  0) 0) |
| [16] |
Deng Y, Fu S, Liang F, et al. Effects of stocking density, diet, and water exchange on growth and survival of pearl oyster Pinctada maxima larvae[J]. Aquaculture International, 2013, 21(6): 1185-1194. DOI:10.1007/s10499-013-9622-0
(  0) 0) |
| [17] |
杨智鹏, 于红, 于瑞海, 等. 不同温度、盐度和培育密度对脉红螺浮游幼虫生长存活的影响[J]. 中国海洋大学学报(自然科学版), 2015, 45(11): 15-19. Yang Zhipeng, Yu Hong, Yu Ruihai, et al. Effects of temperature, salinity and stocking density on the growth and survival of Veined Rapa Whelk (Rapanavenosa) pelagic larvae[J]. Periodical of Ocean University of China, 2015, 45(11): 15-19. (  0) 0) |
| [18] |
Liu W, Gurneysmith H, Beerens A, et al. Effects of stocking density, algal density, and temperature on growth and survival of larvae of the basket cockle, Clinocardium nuttallii[J]. Aquaculture, 2010, 299(1): 99-105.
(  0) 0) |
| [19] |
Marshall R, Pearce C M, Mckinley R S. Interactive effects of stocking density and algal feed ration on growth, survival, and ingestion rate of larval geoduck clams[J]. North American Journal of Aquaculture, 2014, 76(3): 265-274. DOI:10.1080/15222055.2014.886645
(  0) 0) |
| [20] |
Gallager S M. Visual observations of particle Manipulation during feeding in larvae of a bivalve mollusk[J]. Bulletin of Marine Science, 1988, 43(3): 344-365.
(  0) 0) |
| [21] |
高霄龙, 李莉, 孔令锋, 等. 养殖密度对毛蚶幼虫生长及存活的影响[J]. 中国水产科学, 2016, 23(1): 233-240. Gao X, Li L I, Kong L, et al. Effect of stocking density on growth and survival of bloody clam(Scapharca subcrenata)larvae[J]. Journal of Fishery Sciences of China, 2016, 23(1): 233-240. (  0) 0) |
| [22] |
Wang W X, Widdows J. Physiological responses of mussel larvae Mytilus edulis to environmental hypoxia and anoxia[J]. Marine Ecology Progress, 1991, 70(3): 223-236.
(  0) 0) |
| [23] |
Torkildsen L, Magnesen T. Hatchery production of scallop larvae (Pecten maximus)-survival in different rearing systems[J]. Aquaculture International, 2004, 12(4-5): 489-507.
(  0) 0) |
| [24] |
Trevallion A, Johnston R, Finlayson D M, et al. Studies in the bivalve, Tellina tenuis da costa. IV. Further experiments in enriched sea water[J]. Journal of Experimental Marine Biology & Ecology, 1973, 11(2): 189-206.
(  0) 0) |
| [25] |
张涛, 阙华勇. 延迟变态对海洋无脊椎动物生长发育的影响[J]. 海洋科学, 2001, 25(6): 28-30. Zheng T, Que H Y. The effects of delayed metamorphosis on growth and development of invertebrates[J]. Marine Sciences, 2001, 25(6): 28-30. DOI:10.3969/j.issn.1000-3096.2001.06.010 (  0) 0) |
| [26] |
Robert R, Vignier J, Petton B. Influence of feeding regime and temperature on development and settlement of oyster Ostrea edulis (Linnaeus, 1758) larvae[J]. Aquaculture Research, 2007, 48(9): 4756-4773.
(  0) 0) |
| [27] |
Liu B, Dong B, Tang B, et al. Effect of stocking density on growth, settlement and survival of clam larvae, Meretrix meretrix[J]. Aquaculture, 2006, 258(1): 344-349.
(  0) 0) |
| [28] |
Huaiping Z, Guofan Z, Xiao L. Comparison of growth and survival of larvae among different shell color stocks of bay scallop Argopecten irradians irradians(Lamarck 1819)[J]. Chinese Journal of Oceanology and Limuology, 2005, 23(2): 183-188. DOI:10.1007/BF02894236
(  0) 0) |
| [29] |
丛日浩, 李琪, 葛建龙, 等. 长牡蛎4种壳色家系子代的表型性状比较[J]. 中国水产科学, 2014, 21(3): 494-502. Cong R H, Li Q, Ge J L, et al. Comparison of phenotypic traits of four shell color families of the Pacific oyster (Crassostrea gigas)[J]. Journal of Fishery Sciences of China, 2014, 21(3): 494-502. (  0) 0) |
| [30] |
Wolff M, Garrido J. Comparative study of growth and survival of two color morphs of the Chilean scallop Argopecten purpuratus Lamarck (1819) in suspended culture[J]. Journal of Shellfish Research, 1991, 10(1): 47-53.
(  0) 0) |
| [31] |
王庆志, 李琪, 孔令锋, 等. 长牡蛎第三代选育群体生长性状的选择效应[J]. 水产学报, 2013, 37(10): 1487-1494. Wang Q, Qi LI, Kong L, et al. Third generation evaluation of the Pacific oyster (Crassostrea gigas) breeding lines selected for fast growth[J]. Journal of Fisheries of China, 2013, 37(10): 1487-1494. (  0) 0) |
| [32] |
Nell J A, Perkins B. Evaluation of progeny of fourth generation Sydney rock oyster Saccostrea glomerata (Gould, 1850) breeding lines[J]. Aquaculture Research, 2015, 36(8): 753-757.
(  0) 0) |
| [33] |
He M, Guan Y, Yuan T, et al. Realized heritability and response to selection for shell height in the pearl oyster Pinctada fucata, (Gould)[J]. Aquaculture Research, 2008, 39(8): 801-805. DOI:10.1111/j.1365-2109.2008.01889.x
(  0) 0) |
| [34] |
Liang J, Zhang G, Zheng H. Divergent selection and realized heritability for growth in the Japanese scallop, Patinopecten yessoensis Jay[J]. Aquaculture Research, 2010, 41(9): 1315-1321. DOI:10.1111/are.2010.41.issue-9
(  0) 0) |
| [35] |
Azéma P, Maurouard E, Lamy J B, et al. The use of size and growing height to improve Crassostrea gigas, farming and breeding techniques against OsHV-1[J]. Aquaculture, 2017, 471: 121-129. DOI:10.1016/j.aquaculture.2017.01.011
(  0) 0) |
| [36] |
Dove M C, Nell J A, O'Connor W A. Evaluation of the progeny of the fourth-generation Sydney rock oyster Saccostrea glomerata (Gould, 1850) breeding lines for resistance to QX disease (Marteilia sydneyi) and winter mortality (Bonamia roughleyi)[J]. Aquaculture Research, 2013, 44(11): 1791-1800.
(  0) 0) |
| [37] |
邓传敏, 李琪, 孔令锋, 等. 长牡蛎壳金选育系F4与普通养殖群体摄食和呼吸的比较研究[J]. 中国水产科学, 2016, 23(4): 855-863. Deng C M, Qi L I, Kong L, et al. Comparison of feeding and respiration of the forth golden shell colorline and normal culture groups of the Pacific oyster (Crassostrea gigas)[J]. Journal of Fishery Sciences of China, 2016, 23(4): 855-863. (  0) 0) |
| [38] |
Bayne B L. Relations between variable rates of growth, metabolic costs and growth efficiencies in individual Sydney rock oysters (Saccostrea commercialis)[J]. Journal of Experimental Marine Biology& Ecology, 2000, 251(2): 185-203.
(  0) 0) |
2. Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266200, China
 2019, Vol. 49
2019, Vol. 49


