2. 辽宁省海洋水产科学研究院 大连市海产贝类种质资源创新利用重点实验室,辽宁 大连 116023;
3. 青岛海洋科学与技术试点国家实验室 海洋渔业科学与食物产出过程功能实验室,山东 青岛 266237
贝类养殖是中国海水养殖业的支柱产业之一,牡蛎是贝类养殖业中产量最高的贝类。长牡蛎(Crassostrea gigas)也称太平洋牡蛎,具有繁殖力高、适应能力强、生长迅速、营养丰富等特点,是中国牡蛎养殖的主导品种之一。为解决二倍体牡蛎在夏季因繁殖而出现品质下降的问题,研究者成功诱导出三倍体牡蛎,因其育性差、繁殖季节品质高,弥补了二倍体牡蛎的夏季市场空缺。但三倍体长牡蛎中也存在一定比例的可育个体,Jouaux等[1]根据育性不同将三倍体长牡蛎分为α与β两种类型,其中α类型性腺中能够形成较多的生殖细胞并发育至成熟配子,而β类型性腺中生殖细胞数量极少,表现为不育。为何三倍体长牡蛎会出现不同育性?其性腺发育的调控机理是怎样的?这些问题一直受到研究者的关注。性腺发育伴随着能量的储存和利用,开展不同育性三倍体长牡蛎性腺发育的能量转换调控机制研究,不仅对于揭示长牡蛎三倍体育性的分子调控机制有重要意义,对牡蛎育种中育性控制也具有重要的应用价值。
5′AMP活化蛋白激酶(AMPK)是有机体和细胞内感应能量水平并调节代谢稳态的重要分子,在能量和物质代谢、细胞增殖、凋亡、自噬等生命活动中发挥着重要调控作用[2]。在代谢压力下AMPK可通过激活ATP产生途径(脂质氧化、葡萄糖摄取和分解等)和阻断ATP消耗途径(糖原、蛋白质和脂肪酸的合成等)调控能量平衡[3]。AMPK是一种异源三聚体,其亚基分别为AMPK α、AMPK β以及AMPK γ,每个亚基都有2个或3个不同的亚型。AMPK在真核生物中具有高度保守的序列结构和功能域[4],其中,AMPK α的苏氨酸(Thr)172位点已被确定为AMPK激活的决定因素和必需的磷酸化位点[5-7]。AMPK作为一种“能量探测器”参与动物的生殖发育。诸多研究表明AMPK基因在人(Homo sapiens)[8]、牛(Bos taurus)[9]、猪(Sus scrofa)[10]、啮齿动物[11-12]、鸟类[13-14]、长牡蛎[15]和秀丽隐杆线虫(Caenorhabditis elegans)[16]等不同物种的生殖系统中表达,并可能在连接性腺轴与能量平衡的生殖功能中发挥关键作用。Tosca等[17]研究发现牛卵巢中,AMPK激活降低了卵巢颗粒细胞的生长和蛋白质合成;药理激活AMPK可阻止猪和牛的卵母细胞减数分裂恢复[18-19]。此外,Kayampilly和Menon观察到大鼠颗粒细胞暴露于AMPK激活剂后导致细胞周期抑制剂基因p27kip表达增加[20],同时还发现二氢睾酮(DHT)诱导AMPK激活后抑制了颗粒细胞的有丝分裂[21]。
本研究以三倍体长牡蛎为主要研究材料,比较了AMPK三个亚基的mRNA和AMPK α蛋白活性在不同育性长牡蛎雌、雄性腺以及性腺不同发育阶段中的表达模式,探究AMPK基因在长牡蛎不同育性三倍体性腺发育能量调控中的作用,旨在为揭示三倍体长牡蛎性腺发育的分子调控机制提供重要信息。
1 材料与方法 1.1 材料实验材料为二龄长牡蛎,三倍体长牡蛎为二、四倍体杂交产生,二倍体长牡蛎为养殖长牡蛎,二、三倍体长牡蛎均取自荣成市桑沟湾海区,取样时间为4月—6月,每月取二倍体50个,三倍体100个。
1.2 实验方法 1.2.1 倍性检测根据文献[22]的方法,取2~5根鳃丝放入1×PBS缓冲液中剪碎,300目筛绢过滤至预冷的无水乙醇中,4 ℃冰箱过夜沉降。吸取沉淀加到离心管中,PBS缓冲液重悬细胞,用荧光染料碘化丙啶(Propidium lodide,PI)对细胞DNA染色,采用流式细胞仪(Beckman CytoFLEX)测定DNA分子荧光强度,峰值为二倍体长牡蛎的1.5倍视为三倍体。
1.2.2 组织学分析根据文献[22]的方法,取约绿豆粒大小的性腺组织置于10倍体积的Bouin’s固定液中固定24 h,75 %酒精置换之后梯度脱水、透明、石蜡包埋切片,用苏木精-曙红(H.E)染色法染色,封片晾干后用光学显微镜(Olympus BX53)观察并拍照。根据性腺成熟度划分为增殖期(Stage 1)、生长期(Stage 2)和成熟期(Stage 3),对于α和β类型三倍体的判定参照Jouaux等[1],其中3nα为可育型,3nβ为不可育型。
1.2.3 长牡蛎性腺总RNA提取取黄豆粒大小的性腺组织样品放入无酶离心管中,加入1 mL TRIzol试剂,将组织搅碎匀浆,加入200 μL氯仿;12 000 r/min 4 ℃,离心15 min;取上层水相,加入400 μL异丙醇;12 000 r/min 4 ℃,离心10 min;倒掉上层液体,加入预冷过的75 %乙醇1 mL,7 500 r/min 4 ℃,离心5 min;倒掉上层乙醇溶液,干燥10~15 min;加30~50 μL DEPC水溶解RNA,检测RNA浓度和纯度,于-80 ℃保存。
1.2.4 cDNA合成利用反转录试剂盒PrimeScript® RT reagent Kit with gDNA Eraser(TAKARA)将RNA反转录成cDNA第一条链,所得cDNA于-20 ℃保存。
1.2.5 引物设计与合成荧光定量分析所用引物序列如表 1所示,使用EF 1α基因作为内参基因[23],对AMPK基因在不同倍性长牡蛎性腺组织的表达量进行测定。为探究AMPK基因在长牡蛎性腺发育过程中是否参与调节糖原或脂质代谢,我们还选取了糖、脂代谢相关的AMPK潜在靶基因:Raptor、GCS、CREB[15]、ACC[15]和SREBP1[15]进行表达量分析。Raptor、GCS引物均使用Primer Premier 5.0设计,其余引物序列引自文献[15]。
|
|
表 1 荧光定量引物 Table 1 Primers used for qRT-PCR |
使用SYBR© Premix Ex TaqTM Ⅱ Kit,通过实时荧光定量PCR仪器(Roche,LightCycler 480 II)进行基因的相对表达量测定。每组设置6个生物学重复。
1.2.7 蛋白免疫印迹(Western blotting)性腺组织用预冷的PBS缓冲液洗净,加入裂解液后进行匀浆,匀浆管放置冰上裂解30 min,12 000 r/min 4 ℃离心10 min,收集上清总蛋白溶液,加入上样缓冲液后沸水浴变性15 min,电泳,按照Phospho-AMPK α(Thr172)(CST,#2535)抗体说明书进行操作;最后进行化学发光。
1.2.8 免疫组织化学4%多聚甲醛(PFA)固定液固定12 h,甲醇置换3次去除固定液。按照石蜡切片常规流程脱水、透明、包埋和切片。石蜡切片脱蜡。按照Phospho-AMPK α(Thr172)(40H9)Rabbit mAb(CST,#2535)抗体说明书进行实验操作,脱水封片后用光学显微镜(Olympus BX51)观察并拍照。
1.2.9 数据处理与统计根据2-ΔΔCt法得到基因相对表达量数据。实验数据采用SPSS 21.0软件进行统计分析,用单因素方差分析(One-Way ANOVA)对差异表达进行显著性检验,多重比较采用Dunnett’s T3检验,显著水平为P < 0.05。
2 结果 2.1 AMPK基因在长牡蛎性腺发育过程中的表达荧光定量PCR结果显示,AMPK α/β/γ基因mRNA在二、三倍体长牡蛎性腺发育过程中都有表达,在二倍体中三个基因随性腺发育表达量下降,而三倍体中表达趋势相反(见图 1)。二倍体中,AMPK α和AMPK β在增殖期的表达量显著高于成熟期,AMPK γ在雌性中增殖期的表达量显著高于成熟期,雄性中增殖期和成熟期表达量变化不显著。三倍体长牡蛎在增殖期时无法区分雌、雄和育性,与同时期的二倍体相比,三倍体增殖期3个AMPK基因表达量均低于二倍体,其中AMPK α和AMPK γ差异显著(P < 0.05)。成熟期中,AMPK α基因在3nβ的雌性和雄性中表达量较增殖期均显著升高,在3nα雄性中的表达量较增殖期显著升高(P < 0.05);AMPK β在3nβ雄性个体中,表达量较增殖期显著升高(P < 0.05);AMPK γ的表达趋势与AMPK α类似。
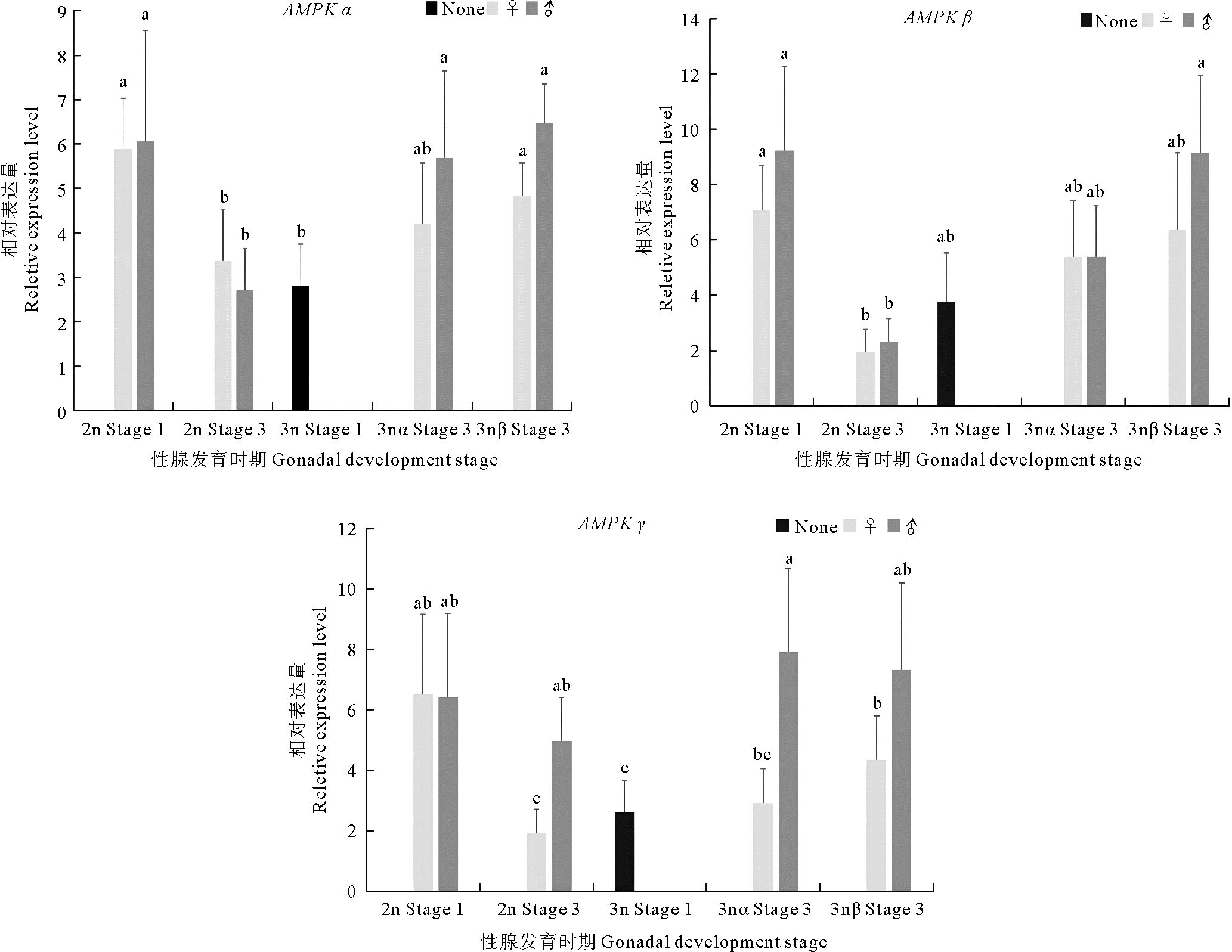
|
(Stage 1:增殖期;Stage 3:成熟期;2n:二倍体;3n:三倍体;3nα:三倍体α;3nβ:三倍体β; None: 不分雌雄; ♀: 雌性; ♂: 雄性。所有的结果都用平均值±标准差(mean±SD)来表示, n=6;同一图中的柱形图上不同的字母(a、b、c)表示差异显著(P < 0.05)。Stage 1: Proliferative stage; Stage 3: Mature stage; 2n: Diploid; 3n: Triploid; 3nα: Triploid α; 3nβ: Triploid β; None: Unable to distinguish between male and female; ♀: Female; ♂: Male. All the results were expressed as mean±SD, n=6; different letters (a、b、c) on the column chart in the same figure indicate significant differences(P < 0.05). ) 图 1 AMPK α/β/γ基因mRNA在长牡蛎性腺发育过程中的表达 Fig. 1 Expression levels of AMPK α/β/γ mRNA during gonadal development of C. gigas |
利用蛋白免疫印迹法分析AMPK α磷酸化水平(p-AMPK α),结果显示二倍体长牡蛎p-AMPK α在增殖期最高,雌性显著高于雄性(P < 0.05),到成熟期时,p-AMPK α水平显著降低(P < 0.05),雄性几乎检测不到磷酸化水平(见图 2)。三倍体增殖期p-AMPK α水平较高,3nα型长牡蛎生长期和成熟期中p-AMPK α水平显著下降(P < 0.05),成熟期几乎检测不到磷酸化(见图 3);3nβ型长牡蛎生长期p-AMPK α水平显著增加,且显著高于3nα型,在成熟期依然存在磷酸化水平,显著高于同时期的二倍体和3nα型。
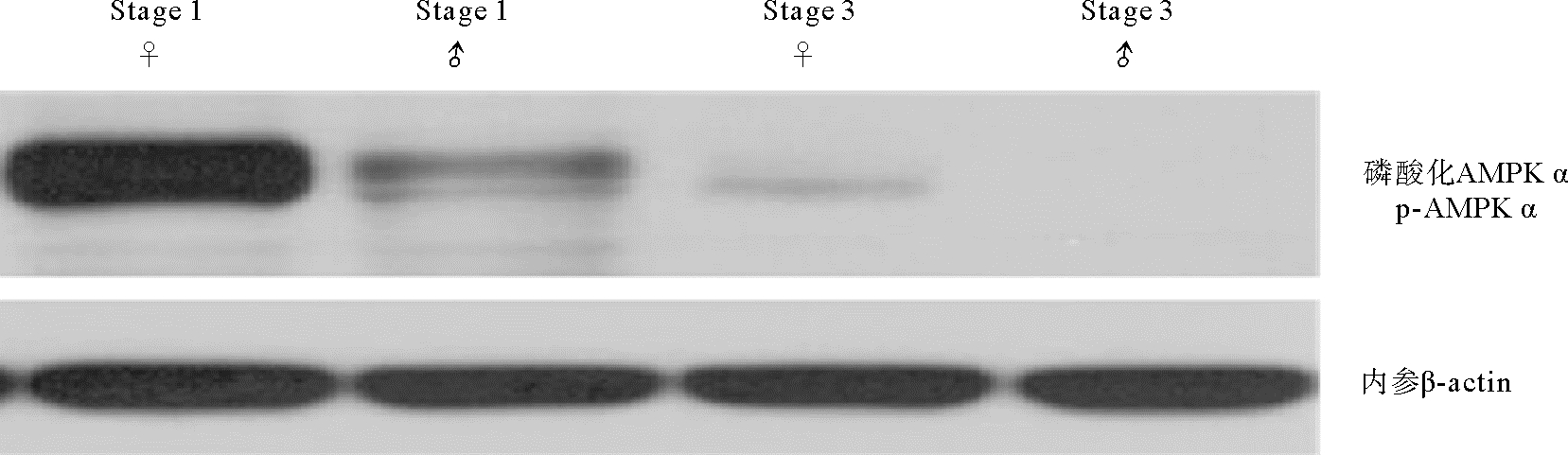
|
(Stage 1:增殖期;Stage 3:成熟期;♀: 雌性; ♂: 雄性。Stage 1: Proliferative stage; Stage 3: Mature stage; ♀: Female; ♂: Male. ) 图 2 二倍体长牡蛎性腺发育过程中AMPK α Thr172磷酸化水平 Fig. 2 Thr172 phosphorylation of AMPK α during gonadal development in diploid C. gigas |
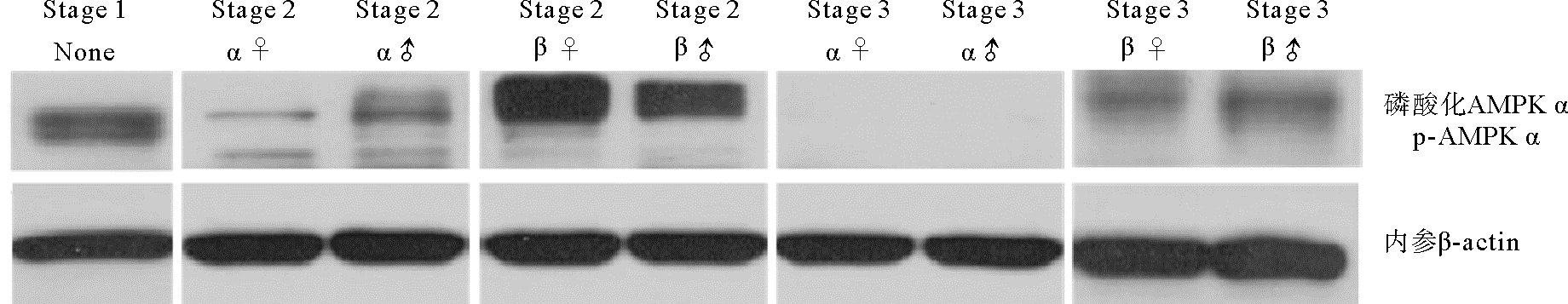
|
(Stage 1:增殖期;Stage 2 α:生长期α;Stage 2 β:生长期β;Stage 3 α:成熟期α;Stage 3 β:成熟期β;None: 不分雌雄; ♀: 雌性; ♂: 雄性。Stage 1: Proliferative stage; Stage 2 α: Development stage α; Stage 2 β: Development stage β; Stage 3 α: Mature stage α; Stage 3 β: Mature stage β; None: Unable to distinguish between male and female; ♀: Female; ♂: Male. ) 图 3 不同育性三倍体长牡蛎性腺发育过程中AMPK α Thr172磷酸化水平 Fig. 3 Thr172 phosphorylation of AMPK α during gonadal development in different types of triploid C. gigas |
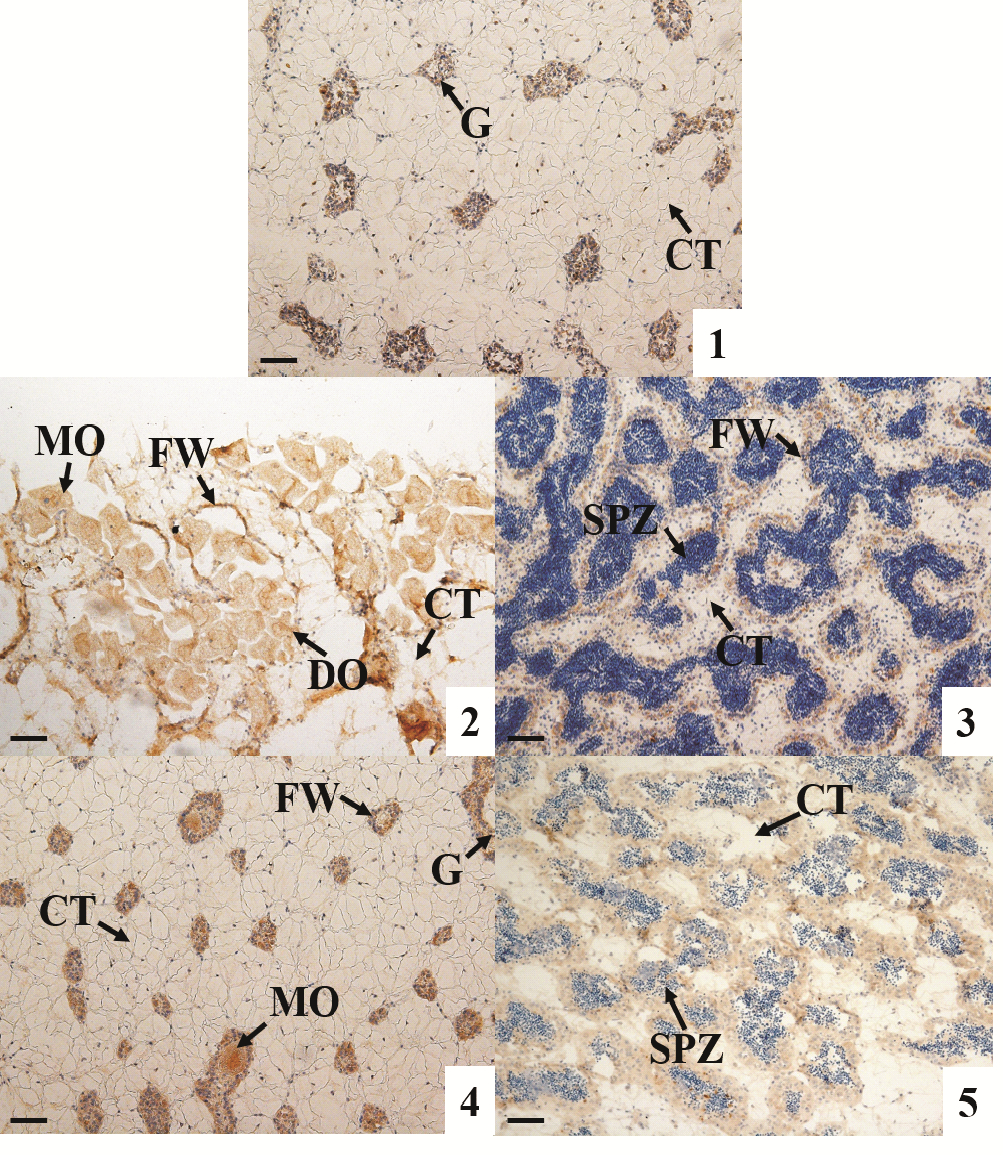
采用免疫组织化学技术检测AMPK α活性蛋白在长牡蛎性腺中的表达情况。结果显示:AMPK α活性蛋白在卵子发生的各时期均有表达,随着卵细胞的逐渐成熟,其免疫染色强度降低,至成熟期卵细胞的免疫染色最弱(见图 4的1,3,5);在雄性的精原细胞和精母细胞中也检测到明显信号,但在精细胞和精子中AMPK α蛋白的表达非常弱,几乎检测不到(见图 4的2,4,6)。AMPK α蛋白在二倍体、三倍体长牡蛎性腺滤泡细胞中都有表达(见图 4和5)。
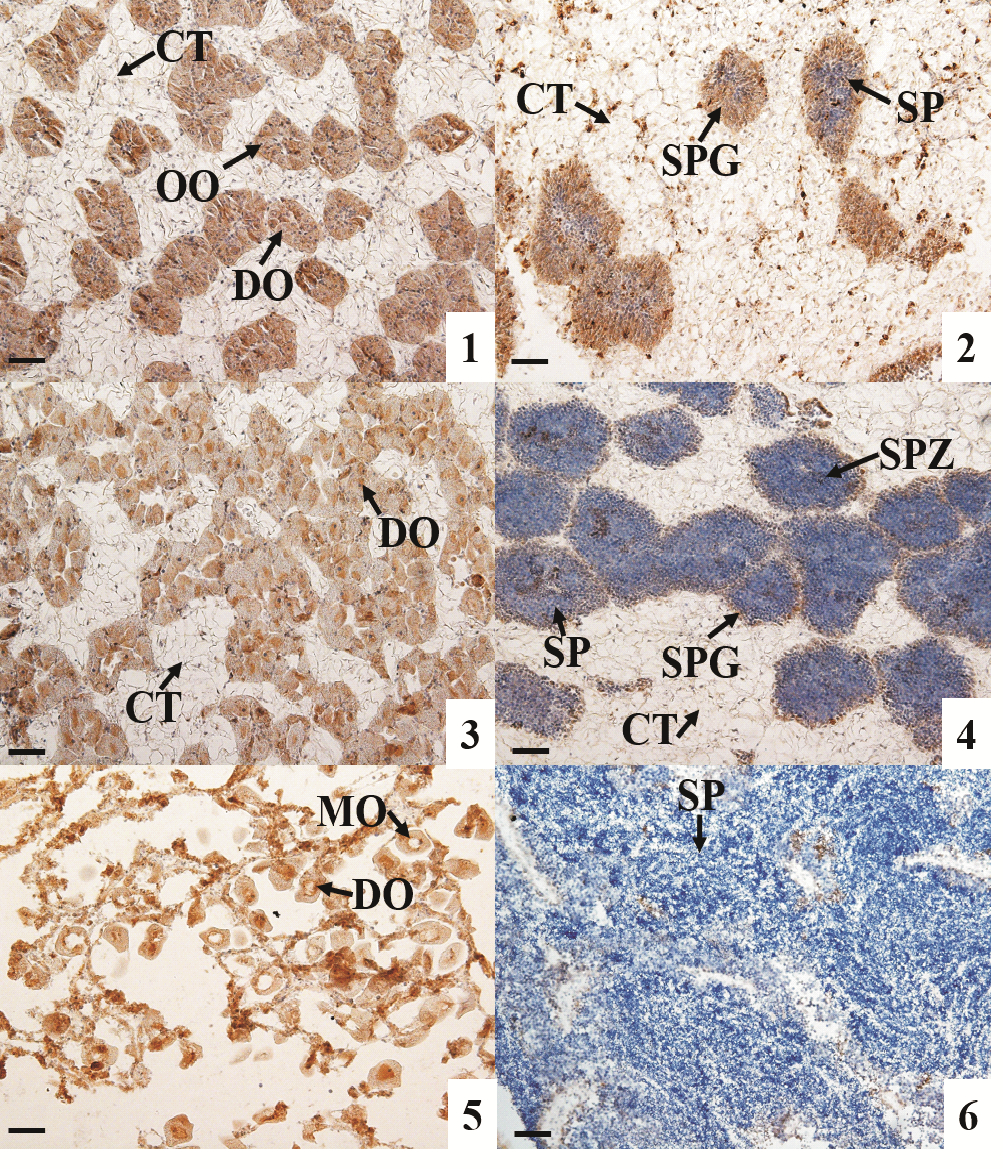
|
(1:增殖期雌性;2:增殖期雄性;3:生长期雌性;4:生长期雄性;5:成熟期雌性;6:成熟期雄性。CT:结缔组织;OO:卵原细胞;DO:未成熟卵子;MO:成熟卵子;SPG:精原细胞;SP:精细胞;SPZ:精子。棕色为阳性信号。标尺:50 μm。1: Proliferative stage female; 2: Proliferative stage male; 3: Development stage female; 4: Development stage male; 5: Mature stage female; 6: Mature stage male. CT: Connective tissue; OO: Oogonia; DO: Developing oocyte; MO: Mature oocyte; SPG: Spermatogonia; SP: Spermatid; SPZ: Spermatozoa. Brown refer to positive signals. Scale bar: 50 μm. ) 图 4 二倍体长牡蛎(C. gigas)性腺发育过程中AMPK α蛋白表达情况 Fig. 4 Expression of AMPK α protein during gonadal development in diploid C. gigas |
|
(1:增殖期,不分雌雄;2:成熟期α雌性;3:成熟期α雄性;4:成熟期β雌性;5:成熟期β雄性。FW:滤泡壁;G:性原细胞;CT:结缔组织;DO:未成熟卵子;MO:成熟卵子;SP:精细胞;SPZ:精子。棕色为阳性信号。标尺:50 μm。1: Proliferative stage, unable to distinguish between male and female; 2: Mature stage α female; 3: Mature stage α male; 4: Mature stage β female; 5: Mature stage β male. FW: Follicle wall; G: Gonia; CT: Connective tissue; DO: Developing oocyte; MO: Mature oocyte; SP: Spermatid; SPZ: Spermatozoa. Brown refer to positive signals. Scale bar: 50 μm. ) 图 5 不同育性三倍体长牡蛎(C. gigas)性腺发育过程中AMPK α蛋白表达情况 Fig. 5 Expression of AMPK α protein during gonadal development in different types of triploids C. gigas |
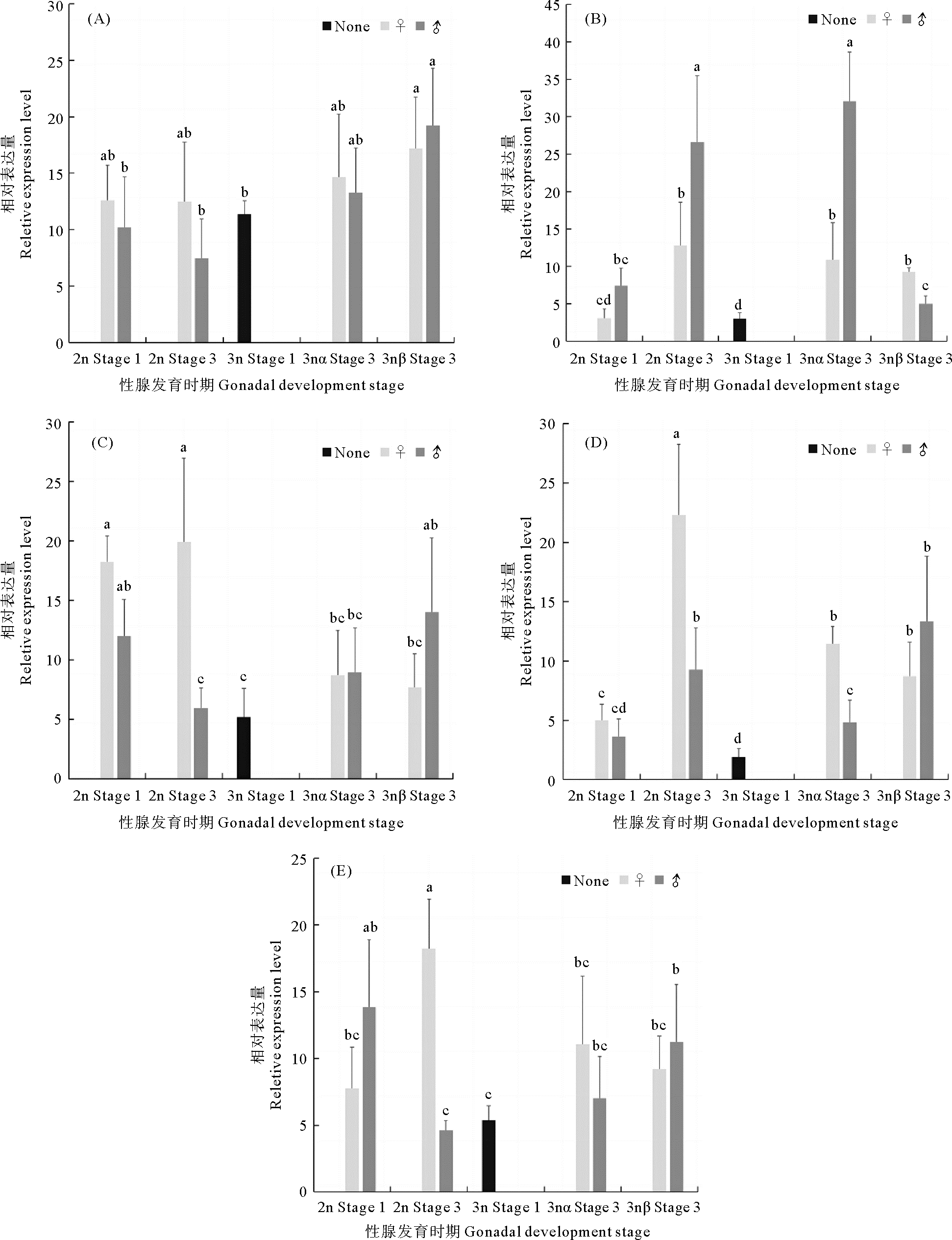
荧光定量PCR结果如图 6所示。糖原合成酶基因GCS mRNA表达量在二倍体和3nα长牡蛎性腺增殖期和成熟期无显著性变化;在3nβ中,成熟期显著高于增殖期(P < 0.05)(见图 6(A))。cAMP反应元件结合蛋白基因(CREB)mRNA在二倍体和三倍体中均是成熟期表达量显著高于增殖期,在成熟期,雌性二倍体、3nα雌性和3nβ雌性间CREB表达量差异不显著,3nβ雄性中CREB表达量显著低于二倍体雄性和3nα雄性(见图 6(B))。乙酰辅酶A羧化酶基因(ACC)mRNA在二倍体雌性长牡蛎性腺发育过程中没有明显变化,在二倍体雄性性腺成熟期显著降低,成熟期雌雄差异显著,雌性显著高于雄性(P < 0.05);在三倍体中,3nα和3nβ ACC表达量差异不显著(见图 6(C))。固醇调节元件结合蛋白基因(SREBP1)在二倍体和三倍体长牡蛎性腺发育过程中的mRNA表达量都是上升的,成熟期表达量显著高于增殖期(P < 0.05),在成熟期时,二倍体和3nα型雌性表达量均显著高于雄性(P < 0.05)(见图 6(D))。mTOR调控相关蛋白基因(Raptor)在二倍体雄性长牡蛎性腺增殖期表达量显著高于成熟期(P < 0.05),在二倍体雌性增殖期表达量显著低于成熟期,且成熟期表达量雌雄差异显著(P < 0.05);在三倍体中的Raptor表达量则呈上升趋势,3nβ雄性表达量显著高于增殖期(见图 6(E))。
|
((A):糖原合成酶;(B):cAMP反应元件结合蛋白;(C):乙酰辅酶A羧化酶;(D):固醇调节元件结合蛋白1;(E):mTOR调控相关蛋白。Stage 1:增殖期;Stage 3:成熟期;2n:二倍体;3n:三倍体;3nα: 三倍体α;3nβ: 三倍体β;None: 不分雌雄;♀:雌性;♂: 雄性。所有的结果都用平均值±标准差(mean±SD)来表示, n=6;不同的字母(a—d)表示差异显著(P < 0.05)。(A): Glycogen synthase; (B): cAMP-response element binding protein; (C): Acetyl-CoA carboxylase; (D): Sterol regulatory element-binding protein l; (E): Regulatory-associated protein of mTOR. Stage 1: Proliferative stage; Stage 3: Mature stage; 2n: Diploid; 3n: Triploid; 3nα: Triploid α; 3nβ: Triploid β; None: Unable to distinguish between male and female; ♀: Female; ♂: Male. All the results were expressed as mean±SD, n=6; different letters (a—d) indicate significant differences(P < 0.05). ) 图 6 AMPK潜在靶基因mRNA在长牡蛎性腺发育过程中的表达 Fig. 6 Expression levels of AMPK putative target genes mRNA during gonadal development of C. gigas |
AMPK α/β/γ基因mRNA在不同倍性长牡蛎性腺发育过程中都有表达。在二倍体中,AMPK α和AMPK β亚基在增殖期性腺的表达量显著高于成熟期,AMPK γ亚基在雌性中增殖期的表达量显著高于成熟期,雄性中增殖期和成熟期表达量变化不显著,说明AMPK α/β/γ亚基可能在长牡蛎性腺发育早期起重要调控作用。三倍体长牡蛎的AMPK α/β/γ亚基mRNA表达趋势与二倍体相反,三倍体增殖期3个亚基表达量均低于二倍体,成熟期时,AMPK α在3nβ和3nα个体中表达量较增殖期明显升高,在3nβ显著升高,AMPK β和AMPK γ在3nβ雄性个体中,表达量较增殖期显著升高。AMPK基因在二倍体、三倍体长牡蛎性腺发育中表达差异可能与二倍体、三倍体性腺发育过程中能量转化及代谢差异密切相关。
AMPK α苏氨酸172(Thr 172)是AMPK激活的决定因子和必需的磷酸化位点[5],只有AMPK α亚基Thr 172位点被磷酸化后才能激活并调节下游目标。二倍体牡蛎性腺p-AMPK α水平在增殖期显著高于成熟期,表明AMPK在增殖期活性高于成熟期。有研究报道,活化的AMPK可以增强葡萄糖的摄取和脂肪酸的氧化分解代谢,抑制糖类、脂类和蛋白质的合成代谢[24]。在长牡蛎的配子发生过程中,需要消耗大量的营养物质用来供给能量和提供配子发育的组成物质,AMPK作为细胞能量稳态的维持者,在二倍体牡蛎增殖期高AMPK α磷酸化水平,可能加强糖原分解、脂质氧化分解和葡萄糖摄取等,产生ATP以供配子发生。3nβ牡蛎性腺生长期的磷酸化水平显著高于可育型,且3nβ在成熟期检测到一定的磷酸化水平,表明AMPK活性的差异可能与三倍体长牡蛎不育相关。AMPK基因被认为会对细胞增殖起负向调控作用,研究表明活化的AMPK会降低大鼠支持细胞的增殖,并增加细胞周期蛋白依赖性激酶抑制剂(CDKI、p19INK4d、p21Cip1和p27Kip1)的表达[25],也会降低颗粒细胞的生长和有丝分裂[17, 21]。3nβ长牡蛎生长期和成熟期相对较高水平的p-AMPK α可能抑制了其配子增殖,从而导致不育。另外,AMPK α磷酸化水平在雌性增殖期中更高,表明AMPK可能在卵子发生过程中具有更广泛的调控作用。AMPK α活性蛋白主要集中在滤泡细胞、卵原细胞、卵母细胞、精原细胞和精母细胞的细胞质和细胞核中,这与哺乳动物的研究结果相似[26],说明AMPK α亚基可能是调控长牡蛎卵原细胞、卵母细胞、精原细胞和精母细胞发育的重要因子。
AMPK可以通过调控下游基因的转录和磷酸化各式底物的方式促进葡萄糖转运和分解代谢、促进脂肪酸氧化、抑制糖异生、抑制脂肪酸和蛋白质合成,从而维持细胞的物质和能量平衡[27-28]。ACC是脂质合成的限速酶基因,在脂质代谢过程中起着重要作用[29],激活的AMPK蛋白可以磷酸化ACC从而降低ACC活性,进而减少脂肪酸合成并提高脂肪酸的氧化量,以适应能量增加的需求[30]。ACC mRNA表达量在二倍体长牡蛎雄性性腺中显著降低,与二倍体AMPK α mRNA和p-AMPK α的变化一致,表明AMPK激活可能正调控雄性长牡蛎性腺中ACC的转录,但与ACC磷酸化程度的调控关系还需后续深入研究。SREBP1基因是细胞内调控脂质代谢的关键转录因子,主要调控胆固醇和脂肪酸合成中关键酶基因的表达,调节脂肪酸和胆固醇合成,AMPK可以抑制SREBP1的表达[31]。本研究中,SREBP1 mRNA表达量变化与AMPK α磷酸化水平变化呈负相关,说明牡蛎性腺中AMPK的激活可能抑制SREBP1的表达,进而抑制脂肪酸和胆固醇合成。CREB是cAMP反应元件CRE的结合蛋白基因,与CRE结合后能大大提高下游基因的转录活性,是糖异生过程中的重要信号转导因子[32],研究表明AMPK通常是调控CREB的共激活因子CRTC2的磷酸化,破坏CREB/CRTC2复合体来影响CREB的功能[33],但在大鼠肌肉和肝脏中AMPK可直接磷酸化并激活CREB[34]。本研究中,二倍体和3nα长牡蛎性腺中CREB基因的表达均在成熟期显著增加,与Guévélou[15]的研究结果一致,AMPK激活可能对CREB的转录表达起到负调控作用。研究发现蛋白质代谢也会受AMPK调控,AMPK可通过磷酸化mTORC1结合Raptor,阻断mTOR信号通路[35],影响蛋白质的翻译合成以及抑制自噬作用等,进而调控细胞的生长和增殖[36]。Raptor基因在二倍体牡蛎雄性性腺中的表达变化趋势与AMPK α mRNA和磷酸化水平变化一致,在雌性性腺中的表达则相反,这提示AMPK可能参与雄性性腺中Raptor基因的转录,推测是由于雄性性腺在增殖期有丝分裂旺盛,需要合成大量蛋白质的原因。靶基因的mRNA表达结果表明,CREB和SREBP1基因与AMPK α mRNA和磷酸化水平存在一定相关性,说明AMPK可能参与调控这两个基因的转录。
AMPK基因在不同育性长牡蛎性腺发育过程中的调控是不一样的。在可育型长牡蛎性腺发育早期,糖原和脂质的大量分解为生殖细胞增殖和分化提供能量,能量过盛导致AMPK基因表达被抑制,从而保证性腺发育过程中持续的能量供给;而在不可育型长牡蛎中,由于性腺发育受阻,不需要大量的能量供给配子发生,因此AMPK并未被抑制,而是发挥能量稳态的调控作用,维持机体的正常生命活动。
| [1] |
Jouaux A, Heude-Berthelin C, Sourdaine P, et al. Gametogenic stages in triploid oysters Crassostrea gigas: Irregular locking of gonial proliferation and subsequent reproductive effort[J]. Journal of Experimental Marine Biology and Ecology, 2010, 395(1-2): 162-170. DOI:10.1016/j.jembe.2010.08.030 (  0) 0) |
| [2] |
Kfoury A, Armaro M, Collodet C, et al. AMPK promotes survival of c-Myc-positive melanoma cells by suppressing oxidative stress[J]. EMBO Journal, 2018, 37(5): e97673. DOI:10.15252/embj.201797673 (  0) 0) |
| [3] |
Bertoldo M J, Faure M, Joëlle D, et al. AMPK: A master energy regulator for gonadal function[J]. Frontiers in Neuroscience, 2015, 9(235): 1-11. DOI:10.3389/fnins.2015.00235 (  0) 0) |
| [4] |
Hardie D G, John W S, David A P. Management of cellular energy by the AMP-activated protein kinase system[J]. FEBS Letters, 2003, 546: 113-120. DOI:10.1016/S0014-5793(03)00560-X (  0) 0) |
| [5] |
Hawley S A, Davison M, Woods A, et al. Characterization of the AMP-activated protein kinase kinase from rat liver and identifi-cation of Threonine 172 as the major site at which it phosphory-lates AMP-activated protein kinase[J]. Journal of Biological Chemistry, 1996, 271(44): 27879-27887. DOI:10.1074/jbc.271.44.27879 (  0) 0) |
| [6] |
Neumann D. Mammalian AMP-activated protein kinase: functional, heterotrimeric complexes by co-expression of subunits in Escherichia coli[J]. Protein Expression and Purification, 2003, 30: 230-237. DOI:10.1016/S1046-5928(03)00126-8 (  0) 0) |
| [7] |
Suter M, Riek U, Tuerk R, et al. Dissecting the role of 5'-AMP for allosteric stimulation, activation, and eactivation of AMP-activated protein kinase[J]. Journal of Biological Chemistry, 2006, 281: 32207-32216. DOI:10.1074/jbc.m606357200 (  0) 0) |
| [8] |
Jane P L, Suman R, Diane M H. Phosphorylation and activation of AMP-activated protein kinase (AMPK) by metformin in the human ovary requires insulin[J]. Endocrinology, 2011, 152(3): 1112-1118. DOI:10.1210/en.2009-1429 (  0) 0) |
| [9] |
Tosca L, Chabrolle C, Uzbekova S, et al. Effects of metformin on bovine granulosa cells steroidogenesis: Possible involvement of adenosine 5'-monophosphate-activated protein kinase (AMPK)[J]. Biology of Reproduction, 2007, 76(3): 368-378. DOI:10.1095/biolreprod.106.055749 (  0) 0) |
| [10] |
Hurtado d L A, Martin-Hidalgo D, Gil M C, et al. New insights into transduction pathways that regulate boar sperm function[J]. Theriogenology, 2016, 85(1): 12-20. DOI:10.1016/j.theriogenology.2015.05.008 (  0) 0) |
| [11] |
Lucie T, Pascal F, Patricia S, et al. Adenosine 5'-monophosphate-activated protein kinase regulates progesterone secretion in rat granulosa cells[J]. Endocrinology, 2005, 146(10): 4500-4513. DOI:10.1210/en.2005-0301 (  0) 0) |
| [12] |
Downs S M, Ya R, Davis C C. Role of AMPK throughout meiotic maturation in the mouse oocyte: Evidence for promotion of polar body formation and suppression of premature activation[J]. Molecular Reproduction Development, 2010, 77(10): 888-899. DOI:10.1002/mrd.21229 (  0) 0) |
| [13] |
Tosca L, Crochet S, Pascal F, et al. AMP-activated protein kinase activation modulates progesterone secretion in granulosa cells from hen preovulatory follicles[J]. Journal of Endocrinology, 2006, 190(1): 85-97. DOI:10.1677/joe.1.06828 (  0) 0) |
| [14] |
Nguyen T M D, Alves S, Grasseau I, et al. Central role of 5'-AMP-activated protein kinase in chicken sperm functions[J]. Biology of Reproduction, 2014, 91(5): 121. DOI:10.1095/biolreprod.114.121855 (  0) 0) |
| [15] |
Guévélou E, Huvet A, Galindo-Sánchez C E, et al. Sex-specific regulation of AMP-activated protein kinase (AMP) in the Pacific oyster Crassostrea gigas[J]. Biology of Reproduction, 2013, 89(4): 1-15. DOI:10.1095/biolreprod.113.109728 (  0) 0) |
| [16] |
Lee H, Cho J S, Lambacher N, et al. The Caenorhabditis elegans AMP-activated protein kinase AAK-2 is phosphorylated by LKB1 and is required for resistance to oxidative stress and for normal motility and foraging behavior[J]. Journal of Biological Chemistry, 2008, 283(22): 14988-14993. DOI:10.1074/jbc.M709115200 (  0) 0) |
| [17] |
Tosca L, Rame C, Chabrolle C, et al. Metformin decreases IGF1-induced cell proliferation and protein synthesis through AMP-activated protein kinase in cultured bovine granulosa cells[J]. Reproduction, 2010, 139: 409-418. DOI:10.1530/rep-09-0351 (  0) 0) |
| [18] |
Lucie T, Svetlana U, Christine C, et al. Possible role of 5'AMP-activated protein kinase in the Metformin-mediated arrest of bovine oocytes at the germinal vesicle stage during in vitro maturation[J]. Biology of Reproduction, 2007, 77(3): 452-465. DOI:10.1095/biolreprod.107.060848 (  0) 0) |
| [19] |
Santiquet N, Sasseville M, Laforest M, et al. Activation of 5' adenosine monophosphate-activated protein kinase blocks cumulus cell expansion through inhibition of protein synthesis during in vitro maturation in swine[J]. Biology of Reproduction, 2014, 91: 1-12. DOI:10.1095/biolreprod.113.116764 (  0) 0) |
| [20] |
Kayampilly P P, Menon K M J. Follicle-stimulating hormone inhibits adenosine 5'-monophosphate-activated protein kinase activation and promotes cell proliferation of primary granulosa cells in culture through an Akt-dependent pathway[J]. Endocrinology, 2009(2): 929-935. DOI:10.1210/en.2008-1032 (  0) 0) |
| [21] |
Kayampilly P P, Menon K M J. AMPK activation by dihydrotestosterone reduces FSH-stimulated cell proliferation in rat granulosa cells by inhibiting ERK signaling pathway[J]. Endocrinology, 2012, 153(6): 2831-2838. DOI:10.1210/en.2011-1967 (  0) 0) |
| [22] |
王朔, 薛茗元, 杨琼, 等. 不同育性三倍体长牡蛎性腺发育过程中的营养成分比较[J]. 水产学报, 2021, 45(1): 88-97. Wang S, Xue M Y, Yang Q, et al. Comparison of nutritional components of different fertility triploid Pacific oyster (Crassostrea gigas) during gonadal development[J]. Journal of Fisheries of China, 2021, 45(1): 88-97. (  0) 0) |
| [23] |
Hu B, Li Q, Yu H, et al. Identification and characterization of key haem pathway genes associated with the synthesis of porphyrin in Pacific oyster (Crassostrea gigas)[J]. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2021, 255: 1-12. DOI:10.1016/j.cbpb.2021.110595 (  0) 0) |
| [24] |
杨航, 杨吉春, 管又飞. AMPK在机体糖脂代谢中的作用[J]. 生理科学进展, 2009, 40(3): 249-252. Yang H, Yang J C, Guan Y F. Role of AMPK in glucose and lipid metabolisms[J]. Progress in Physiological Sciences, 2009, 40(3): 249-252. (  0) 0) |
| [25] |
Riera M F, Regueira M, Galardo M N, et al. Signal transduction pathways in FSH regulation of rat Sertoli cell proliferation[J]. American Journal of Physiology Endocrinology & Metabolism, 2012, 302(4): 914-923. DOI:10.1152/ajpendo.00477.2011 (  0) 0) |
| [26] |
Tosca L, Chabrolle C, Dupont J. AMPK: A link between metabolism and reproduction?[J]. Medicine Sciences, 2008, 24(3): 297-300. DOI:10.1051/medsci/2008243297 (  0) 0) |
| [27] |
Herzig S, Shaw R J. AMPK: Guardian of metabolism and mitochondrial homeostasis[J]. Nature Reviews Molecular Cell Biology, 2018, 19(2): 121-135. DOI:10.1038/nrm.2017.95 (  0) 0) |
| [28] |
丁婕, 傅继华. AMPK、PPARs在2型糖尿病中的作用机制及药物研究[J]. 药学研究, 2019, 38(8): 477-480, 389. Ding J, Fu J H. Mechanism and drug research of AMPK and PPARs in type 2 diabetes mellitus[J]. Journal of Pharmaceutical Research, 2019, 38(8): 477-480, 389. (  0) 0) |
| [29] |
Hardie D G, Pan D A. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase[J]. Biochemical Society Transactions, 2002, 30(6): 1064-1070. DOI:10.1042/bst0301064 (  0) 0) |
| [30] |
林志健, 张冰, 刘小青. AMPK-ACC信号通路与相关代谢疾病的研究进展[J]. 中国糖尿病杂志, 2013, 21(5): 474-477. Lin Z J, Zhang B, Liu X Q. Advances of researches on AMPK-ACC signaling pathway in metabolic diseases[J]. Chinese Journal of Diabetes, 2013, 21(5): 474-477. (  0) 0) |
| [31] |
Yang J, Craddock L, Hong S, et al. AMP-activated protein kinase suppresses LXR-dependent sterol regulatory element-binding protein-1c transcription in rat hepatoma McA-RH7777 cells[J]. Journal of Cellular Biochemistry, 2009, 106(3): 414-426. DOI:10.1002/jcb.22024 (  0) 0) |
| [32] |
Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB[J]. Nature Reviews Molecular Cell Biology, 2001, 2(8): 599-609. DOI:10.1038/35085068 (  0) 0) |
| [33] |
唐红菊, 张玉青, 王晓, 等. 二甲双胍抑制肝脏糖异生的机制研究[J]. 中华内分泌代谢杂志, 2013, 29(11): 971-976. Tang H J, Zhang Y Q, Wang X, et al. On the mechanism of metformin-inhibited hepatic gluconeogenesis[J]. Chinese Journal of Endocrinology and Metabolism, 2013, 29(11): 971-976. (  0) 0) |
| [34] |
Thomson D M, Herway S T, Fillmore N, et al. AMP-activated protein kinase phosphorylates transcription factors of the CREB family[J]. Journal of Applied Physiology, 2008, 104(2): 429-438. DOI:10.1152/japplphysiol.00900.2007 (  0) 0) |
| [35] |
符庆瑛, 高钰琪. 蛋白激酶AMPK的研究进展[J]. 生命科学, 2005, 17(2): 147-152. Fu Q Y, Gao Y Q. Advances in the studies of AMP-activated protein kinase[J]. Chinese Bulletin of Life Sciences, 2005, 17(2): 147-152. (  0) 0) |
| [36] |
Polak P, Hall M N. mTOR and the control of whole body metabolism[J]. Current Opinion in Cell Biology, 2009, 21(2): 209-218. DOI:10.1016/j.ceb.2009.01.024 (  0) 0) |
2. Dalian Key Laboratory of Genetic Resources for Marine Shellfish, Liaoning Ocean and Fisheries Science Research Institute, Dalian 116023, China;
3. Laboratory for Marine Fisheries Science and Food Production Processes, Pilot National Laboratory for Marine Science and Technology(Qingdao), Qingdao 266237, China
 2024, Vol. 54
2024, Vol. 54


