2. 青岛农业大学海洋科学与工程学院,山东 青岛 266237;
3. 海南热带海洋学院水产与生命学院,海南 三亚 572022
紫菜是海水养殖中最重要的经济红藻,年产量估计超过100 000 t(干质量),产值约13亿美元[1]。坛紫菜(Pyropia haitanensis)隶属于红藻门(Rhodophyta),红毛菜纲(Bangiophyceae),红毛菜目(Bangiales),红毛菜科(Bangiaceae),紫菜属(Pyropia),是紫菜养殖的主要种类[2]。作为生活在潮间带的大型红藻,其生活环境包含长时间且高强度的失水胁迫。在退潮时,许多紫菜在藻体失水率达80%可以存活,甚至有些紫菜在藻体失水率达95%的情况下依旧可以存活,并在涨潮后可以完全恢复其生理机能[3]。紫菜中存在多种对抗渗透压胁迫的机制,是耐失水能力最强的海藻之一。因此,可以作为研究潮间带海藻耐受渗透胁迫的分子机制的理想模式生物[4]。已有研究发现:坛紫菜在退潮失水时会发生一些生理变化,如光合活性降低,抗氧化能力增强,细胞含水量、细胞壁结构和细胞体积发生改变,以此降低失水胁迫带来的损害[5]。
代谢组(Metabolome)是生物系统中小分子的完整集合[6]。当植物暴露于非生物胁迫时,可以通过调节由基因-蛋白质-代谢物-表型组成的复杂调控网络来维持体内平衡,因此代谢物谱通常被认为是连接表型和基因型的桥梁,并且可以反映生长中植物的生理状态[7-8]。代谢组学(Metabolomics)分析主要目的是从生物样本中检测并筛选出具有重要生物学意义和统计学显著差异的代谢物,并以此为基础阐明生物体的代谢过程和变化机制[9]。代谢物分析已经广泛运用在小麦[10]、葡萄藤[11]、黑麦草[12]、棉花[13]、马铃薯[14-15]、拟南芥[16]、大豆[17-18]和番茄[19]等植物对干旱胁迫下的响应机制研究中。
目前对于紫菜失水胁迫响应机制的组学研究包括转录组分析[20-21]和蛋白质组分析[22],相关的代谢组研究尚未见报道。本研究基于UPLC-MS/MS检测平台,结合多种统计分析方法,对坛紫菜正常对照组、失水处理和复水处理的代谢组进行了系统研究,填补了坛紫菜抗失水胁迫代谢组分析的空白,为坛紫菜的抗失水胁迫机制及代谢调控机制提供了参考。
1 材料与方法 1.1 实验材料本实验使用的材料是坛紫菜PH40纯系(PHL200812-24),叶型为细长形,其祖先配子体于2010年在中国福建省福鼎的潮间带采集,之后制备了遗传上纯合的PH40[23]。叶状体培养条件如下:光强50~60 μmol photons·m-2·s-1;温度(20±1) ℃;光照周期为12L∶12D。每升海水培养材料鲜质量为1.0~1.5 g,每3天更换一次新鲜含PES培养基[24]的海水,PES培养基配方如表 1所示。
|
|
表 1 PES培养基配方 Table 1 PES medium formula |
|
|
表 2 母液Ⅲ配方 Table 2 ES Fe solution formula |
|
|
表 3 母液Ⅳ培养基配方 Table 3 P-Ⅱ Metal solution formula |
将失水处理的坛紫菜放置于恒温箱中,保持温度、光照条件与叶状体日常相应条件一致。
(1) 提前计算材料含水率C和达到80%失水率的坛紫菜的实际质量Wt,计算公式如下:
| $ \begin{gathered} C=\left(W_{0}-W_{\mathrm{d}}\right) / W_{0}, \\ W_{\mathrm{t}}=W_{0}-L \cdot C \cdot W_{0}。\end{gathered} $ |
式中:W0是坛紫菜鲜质量,Wd是坛紫菜干质量,L为失水率(L=80%),C为含水率。
(2) 失水组处理:从海水中取出坛紫菜叶状体,平铺在无菌玻璃皿上,用无菌纱布轻按藻体快速去除多余水分,之后马上称量得到坛紫菜鲜质量。将平铺在玻璃皿中的坛紫菜暴露在培养箱的空气中,一段时间后称取坛紫菜实时质量,当样品失水率为80%时,迅速用无菌镊子收集样品放入锡箔纸,做好标记,入液氮急冻。
(3) 复水组处理:按照(2)中所述方法,使样品失水率达到80%,之后迅速放入含PES的海水,0.5 h后,用无菌纱布吸干藻体多余水分,收集样品,做好标记,入液氮急冻。
(4) 对照组无处理:直接从海水中取出新鲜叶状体,吸取表面水分后马上收集并进行液氮速冻。
(5) 将所有样品保存在超低温冰箱(-80 ℃)。
本次实验共处理了三组样本,分别为对照组(C)、失水组(D)以及复水组(R),每组三个生物学重复,用于探究坛紫菜在失水胁迫过程中代谢物种类及含量变化。
1.3 坛紫菜不同处理组代谢物提取使用冷冻研磨仪(Tissuelyser-24)在60 Hz条件下对真空冷冻干燥后的样品研磨1 min。然后将每100 mg样品粉末溶解于1.0 mL含有0.1 mg/L利多卡(内标)的70%甲醇溶液中提取代谢物。提取完成后,10 000 g离心10 min,吸取上清,用微孔滤膜(0.22 μm)过滤样品,并保存在进样瓶中以待LC-MS/MS分析。
1.4 质谱检测不同处理组坛紫菜代谢物数据采集仪器系统主要包括超高效液相色谱Shim-pack UFLC SHIMADZU CBM20A串联质谱Applied Biosystems 4500 QTRAP,使用Waters ACQUITY UPLC HSS T3 C18(1.8 μm,2.1 mm·100 mm)色谱柱。
流动相为0.04%乙酸水溶液和乙腈(含0.04%乙酸),流速为0.4 mL/min;柱温为40 ℃;进样量5 μL。电喷雾离子源(Electrospray ionization,ESI)温度为550 ℃,质谱电压为5 500 V,帘气(Curtain gas, CUR)为171.25 kPa。
1.5 数据分析通过软件Analyst 1.6.1对原始数据进行定性定量分析。利用R软件的内置cor函数计算样品间的皮尔逊相关系数(Pearson’s correlation coefficient,r),对样品生物学重复相关性进行评估。将三组数据进行两两比较,使用SIMCA-P14.1软件对主成分进行偏最小二乘判别分析(Partial least squares-discriminant analysis, PLS-DA),寻找差异代谢物。通过R软件(www.r-project.org/)对代谢物在不同样本间的积累模式进行聚类分析(Hierarchical cluster analysis,HCA)。通过MBRole(Metabolites biological role,http://csbg.cnb.csic.es/mbrole/)获取差异代谢物的KEGG ID,并进行KEGG通路富集分析[25]。
2 结果与分析 2.1 三组坛紫菜样品代谢物定性定量分析图 1为多反应离子检测(Multiple reaction monitoring,MRM)代谢物检测多峰图。基于本地代谢数据库MWDB(迈维代谢生物科技有限公司,中国,武汉)(http://www.metware.cn/)数据,对样本的代谢物进行了质谱定性定量分析。图 1展示了样本中能够检测到的物质,每个不同颜色的质谱峰代表检测到的一个代谢物。本实验中共检测到332个代谢物,其中已知的有206个,未知的有126个。
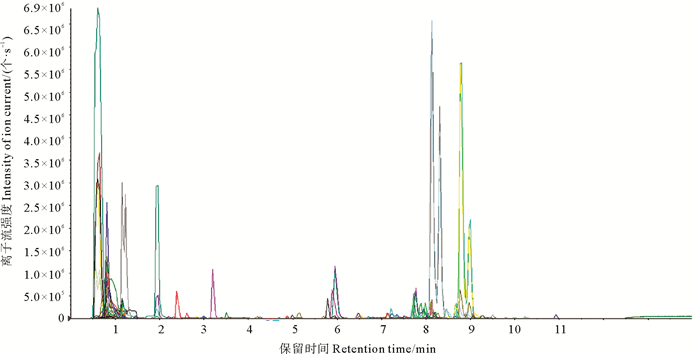
|
图 1 MRM代谢物检测多峰图 Fig. 1 MRM metabolite detection multi peak map |
如图 2所示,色块中的数字表示r2,r2越接近1,两个重复样品相关性越强。坛紫菜三组样品代谢组数据组内平行性较为良好,组间具有一定差异。可以对三组代谢组数据进行进一步分析以筛选差异代谢物。
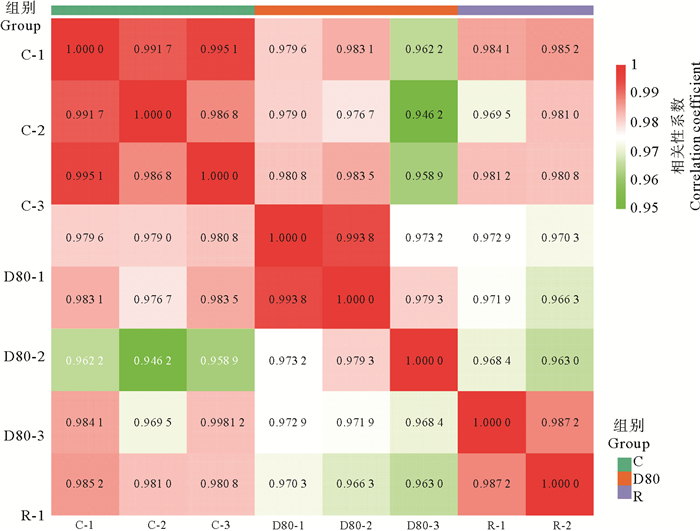
|
( C:坛紫菜对照组;D80:坛紫菜失水处理组;R:坛紫菜复水处理组。图中色块中的数字代表相关系数数值大小。C: Control group of P. haitanensis; D80: Dehydration treatment group of P. haitanensis; R: Rehydration treatment group of P. haitanensis. The number in the color block represents the value of correlation coefficient. ) 图 2 样品间相关性图 Fig. 2 Correlation between samples |
三组样品的代谢组进行聚类热图分析如图 3所示,对照组与复水组的代谢组被聚类到一起,而失水组与对照组和复水组的代谢组存在较大差异。在检出的所有代谢物中,失水组与对照组相比,有2/3左右代谢物呈现上调表达,而在失水组上调的代谢物在复水组多呈现下调。
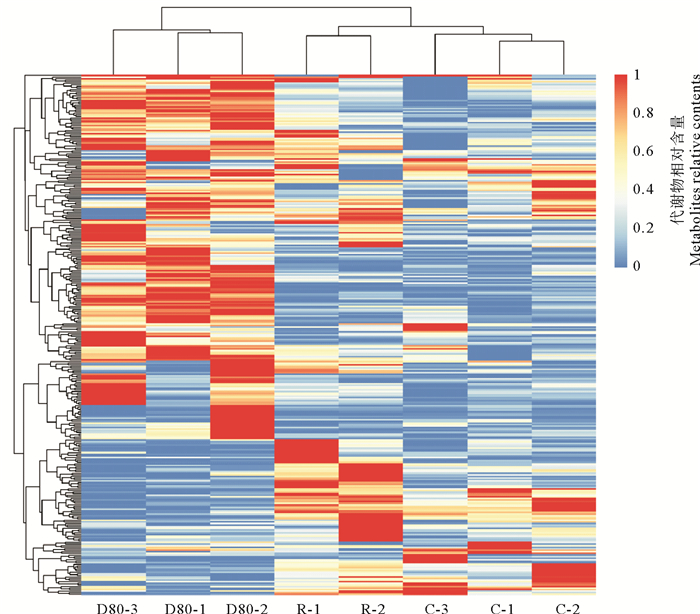
|
( C:坛紫菜对照组;D80:坛紫菜失水处理组;R:坛紫菜复水处理组。图中每行代表一种代谢物,颜色表示代谢物的相对含量,颜色越红表示代谢物在对应组中含量越高。C: Control group of P. haitanensis; D80: Dehydration treatment group of P. haitanensis; R: Rehydration treatment group of P. haitanensis. Each row in the heatmap represents a metabolite. The color represented the relative content of metabolites. The more redder the color, the higher the content of metabolites in the corresponding group. ) 图 3 三组坛紫菜样品代谢物聚类热图分析 Fig. 3 Heatmap showing the variation of metabolites in P. haitanensis |
为了进一步挖掘差异代谢物,作者对数据进行PLS-DA分析(Partial least squares-discriminant analysis, PLS-DA)。如图 4(A)所示,对照组与失水组的PLS-DA得分图显示组间明显分离,且组内明显聚类,两组样品在空间点上可以明显区分,表明两组代谢组间存在差异。其模型质量参数为:主成分1、主成分2、对X矩阵的解释率R2X、对Y矩阵的解释率R2Y、模型的预测能力Q2。其中R2X=0.754,R2Y=1, Q2=0.989。这表明当前PLS-DA模型解释和预测数据能力较好。进而对这两组的PLS-DA进行排列验证(n=200,即进行200次排列实验),如图 3(B)所示:右边的原始Q2、R2均高于左侧对应颜色的实验点,说明可根据VIP值筛选对照组与失水S组代谢组间的差异代谢物。
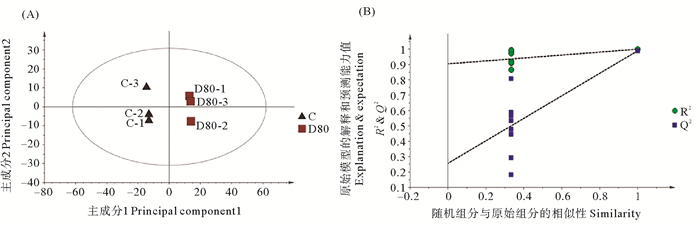
|
( R2:模型对矩阵的解释率,Q2:模型的预测能力。R2: The interpretation rate of the model to the matrix; Q2: The prediction ability of the model. ) 图 4 (A) 坛紫菜对照组C(黑色三角)与失水组D80(红色方框)的PLS-DA得分图;(B) PLS-DA分析模型置换检验图 Fig. 4 (A) PLS-DA scores of P. haitanensis control (black triangle) and dehydration group(red box); (B) permutation test of PLS-DA model |
本研究将VIP≥1.0且差异倍数显著(fold change≥2以及fold change≤0.5)的代谢物定义为差异代谢物。基于此标准在失水组与对照组间筛出58种差异代谢物,如图 5所示。在这58种差异代谢物中,有38种在失水组的含量高于对照组,为上调代谢物,主要包括氨基酸类(甲硫氨酸、色氨酸、半胱氨酸、蛋氨酸等)和氨基酸衍生物(亮氨酸-脯氨酸)、苯丙素类(阿魏酰奎宁酸,反式肉桂酸,桂皮醛以及对香豆酰奎尼酸)、甘油磷脂(溶血卵磷脂酰胆碱)、核苷酸及其衍生物、黄酮类、类黄酮类、色胺、薯蓣皂苷以及植物激素水杨酰咖啡酸;20个下调代谢物中主要包括黄酮醇、类黄酮、萜类、脂肪酸、核苷酸衍生物、脱落酸和反式玉米素,其中最后两种为植物激素。统计学分析结果表明在这58种差异代谢物中有41种代谢物在对照组与失水组间存在显著差异(P<0.05)。
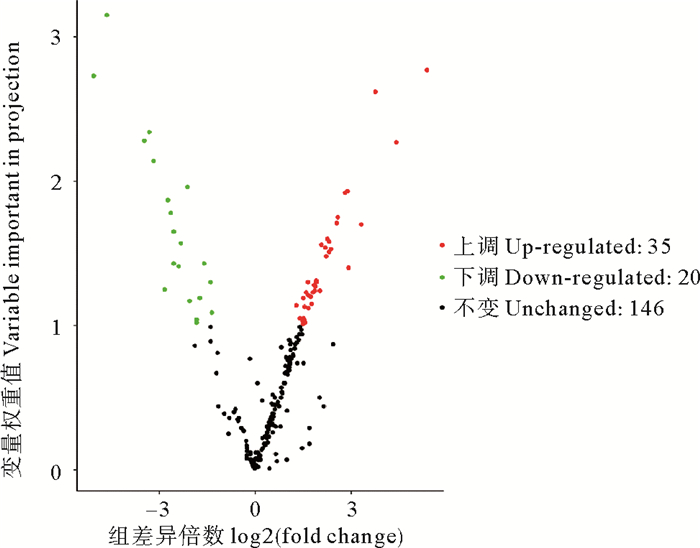
|
图 5 坛紫菜对照组与失水组的差异代谢物火山图 Fig. 5 Volcanic map of different metabolites between control group and dehydration group of P. haitanensis |
对照组与失水组的PLS-DA得分图显示组间明显分离,且组内明显聚类,表明两组代谢组间存在差异,其模型质量参数为:主成分1、主成分2、对X矩阵的解释率R2X、对Y矩阵的解释率R2Y、模型的预测能力Q2。其中R2X=0.871,R2Y =1,Q2=0.999,这表明当前PLS-DA模型解释和预测数据能力较好(见图 6(A))。进而对这两组的PLS-DA进行排列验证(n=200,即进行200次排列实验),右边的原始Q2、R2均高于左侧对应颜色的实验点,说明可根据VIP值筛选对照组与失水组代谢组间的差异代谢物(见图 6(B))。
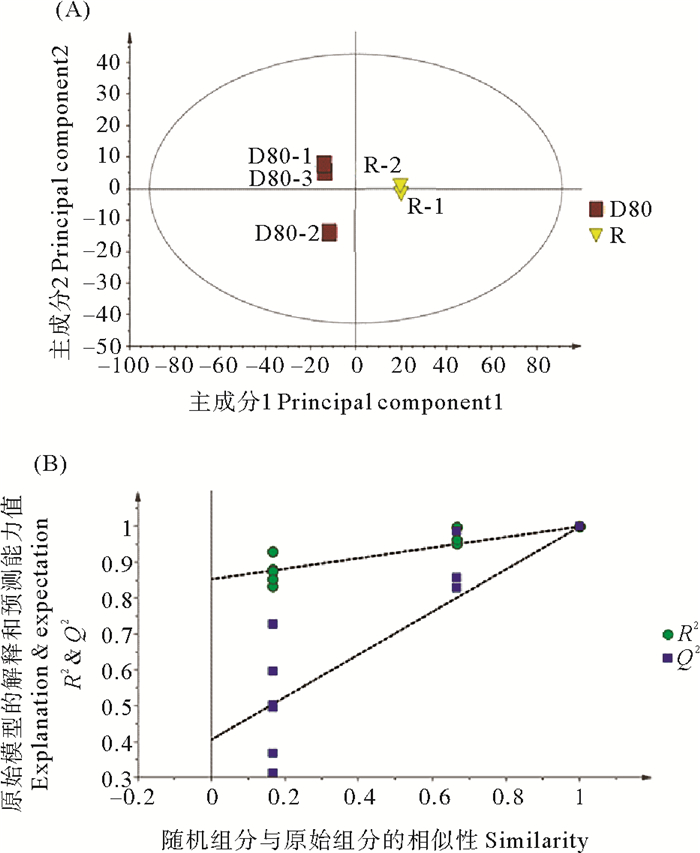
|
( R2:模型对矩阵的解释率,Q2:模型的预测能力。R2: The interpretation rate of the model to the matrix; Q2: The prediction ability of the model. ) 图 6 (A) 坛紫菜复水组R(黄色倒三角)与失水组D80(红方框)的PLS-DA得分图;(B)PLS-DA分析模型置换检验图 Fig. 6 (A) PLS-DA scores of P. haitanensis rehydration group(yellow inverted triangle)and dehydration group (red box); (B)permutation test of PLS-DA model |
在复水组与失水组间按标准筛出50种差异代谢物,如图 7所示。在这50种差异代谢物中,有30种在复水组的含量高于失水组,为上调代谢物,主要包括包括儿茶素及其衍生物、黄酮、黄酮C、黄酮醇、类黄酮、脂肪酸、多种萜类、少量氨基酸衍生物、苯丙素类(绿原酸,白藜芦醇)以及植物激素脱落酸;20个下调代谢物中主要包括甘油磷脂(溶血卵磷脂酰胆碱)、氨基酸、核苷酸及其衍生物、黄酮类、色胺、对香豆酰阿魏酰咖啡酰亚精胺和维生素B2。统计学分析结果表明在这50种差异代谢物中有31种代谢物在复水组与失水组间存在显著差异(P<0.05)。
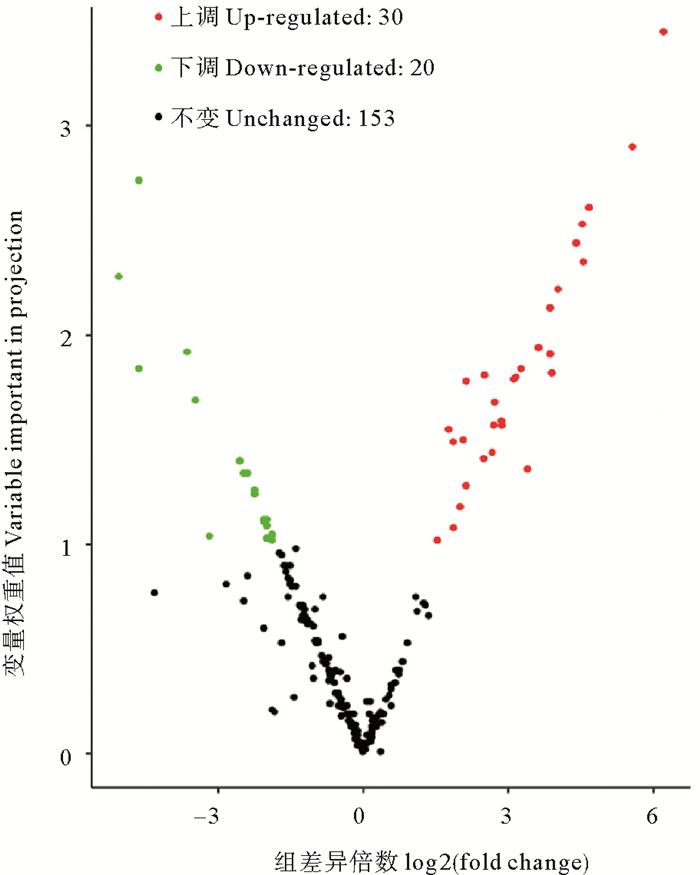
|
图 7 坛紫菜复水组与失水组的差异代谢物火山图 Fig. 7 Volcanic map of different metabolites between rehydration group and dehydration group of P. haitanensis |
如图 8(A)所示,对照组与复水组的PLS-DA得分图显示组间明显分离,且组内明显聚类,表明两组代谢组间存在差异。其模型质量参数为:具有2个主成分,R2X=0.669,R2Y =0.999,Q2=0.937,这表明当前PLS-DA模型解释和预测数据能力较好。进而对这两组的PLS-DA进行排列验证(n=200,即进行200次排列实验),如图 8(B)所示:右边的原始Q2、R2均高于左侧对应颜色的实验点,说明可根据VIP值分析筛选对照组与复水组间的差异代谢物。
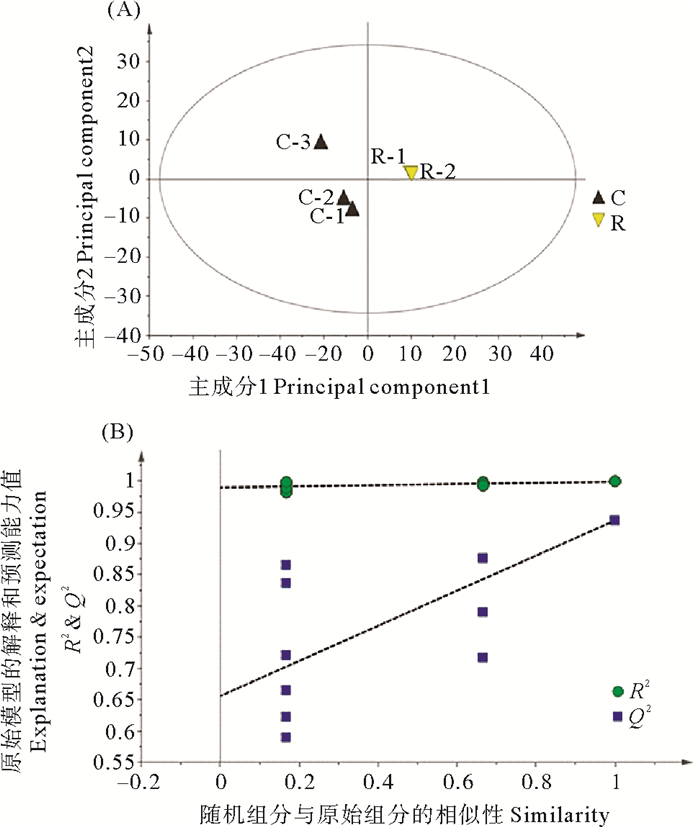
|
( R2:模型对矩阵的解释率,Q2:模型的预测能力。R2: The interpretation rate of the model to the matrix; Q2: The prediction ability of the model. ) 图 8 (A) 坛紫菜对照组D(黑三角)与复水组R(黄色倒三角)的PLS-DA得分图;(B)PLS-DA分析模型置换检验图 Fig. 8 (A) PLS-DA scores of P. haitanensis control(Black triangle) and rehydration group(yellow inverted triangle); (B) permutation test of PLS-DA model |
如图 9所示,按标准在对照组与复水组间共筛选出42种差异代谢物。在这42种差异代谢物种,有31种代谢物在复水组的含量高于对照组,即上调代谢物,包括氨基酸类(丙氨酸、蛋氨酸、丝氨酸、甲硫氨酸等)和氨基酸衍生物、大量儿茶素衍生物(儿茶素,表儿茶素等)、苯丙素类(对香豆酰奎尼酸、绿原酸)、萜类、多种脂肪酸、黄酮/黄酮醇/类黄酮、甜菜碱和D(-)-苏阿糖;11个下调代谢物主要包括黄酮、黄酮C/类黄酮、硫胺素、反式玉米素和锦葵色素-3-葡糖苷。统计学分析结果表明在这42种差异代谢物中有13种代谢物在复水组与失水组间存在显著差异(P<0.05)。
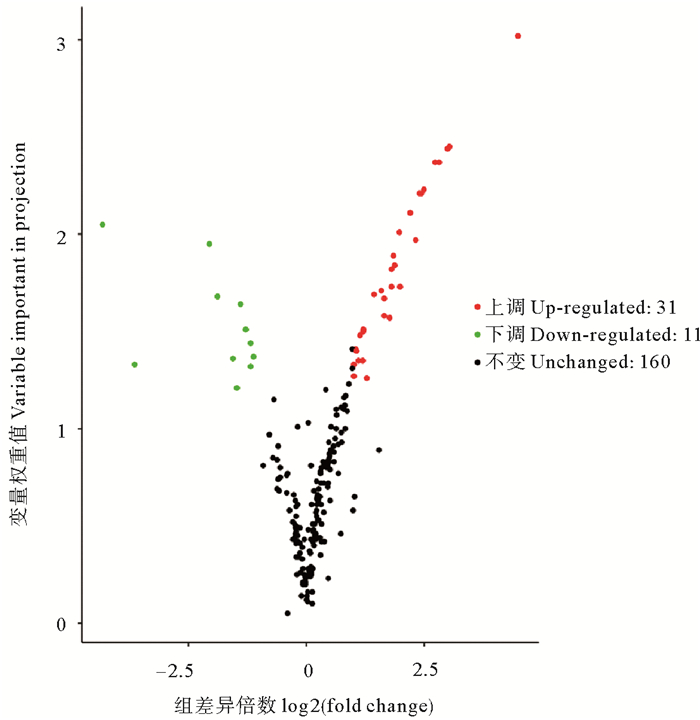
|
图 9 坛紫菜对照组与复水组的差异代谢物火山图 Fig. 9 Volcanic map of different metabolites between control group and rehydration group of P. haitanensis |
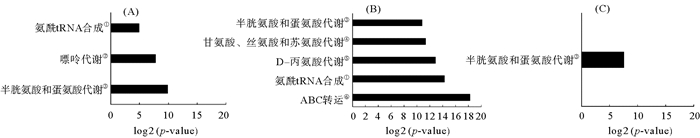
KEGG对坛紫菜失水-复水过程中的差异代谢物的通路富集分析结果如图 10所示。在失水过程富集到3条代谢通路,分别是氨酰-tRNA生物合成、嘌呤代谢以及半胱氨酸和蛋氨酸代谢,这些通路可能与坛紫菜抗失水胁迫机制密切相关(见图 10(A))。图 10(B)表明与对照组相比,复水组中富集到5条代谢通路,分别是半胱氨酸和蛋氨酸代谢、甘氨酸/丝氨酸/苏氨酸代谢、D-丙氨酸代谢、氨酰-tRNA生物合成以及ABC转运。图 10(C)表明坛紫菜在失水又到复水这个过程中,只富集到半胱氨酸和蛋氨酸代谢这一条代谢通路。
|
( A. 坛紫菜对照组与失水组;B坛紫菜对照组与复水组;C坛紫菜复水组与失水组;横坐标是对出现富集的通路的p-value取log2所得值。①Aminoacyl-tRNA biosynthesis; ②Purine metabolism; ③Cysterne and methionine metabolism; ④Clycine, serine and threonine metabolism; ⑤D-Alanine metabolism; ⑥.ABC transporters. A. Control group and dehydration group; B. Control group and rehydration group; C. Rehydration group and dehydration group; The abscissa is log2 for the p-value of the enriched pathway. ) 图 10 坛紫菜不同处理组间差异代谢物代谢通路富集分析 Fig. 10 Enrichment analysis of metabolic pathway of different metabolites between two groups of P. haitanensis |
紫菜是一种生活在上潮间带具有极强抗失水胁迫的大型红藻,可以耐受重度失水,并在复水后迅速恢复生理活性,是研究潮间带红藻的模式物种。代谢物是生物化学活动的直接标志,而基于UPLC-MS/MS技术代谢组学具有高通量、高灵敏度以及定性定量准确的优势,为快速准确检测海藻代谢组提供了技术保障。本研究是针对坛紫菜在失水-复水过程中代谢组的变化进行的分析,有助于理解红藻独特的抗失水胁迫机制。
在本研究中,坛紫菜的失水组、复水组与对照组的代谢组间均有显著差异。在坛紫菜经历失水-复水的过程中,细胞含水量发生剧烈变化,有多种代谢物明显上调,积极响应失水胁迫。黄酮类物质具有很强的抗氧化能力。在小麦叶片中,类黄酮生物合成基因的表达和类黄酮在干旱胁迫下的积累提高了胁迫耐受性[26]。此外,具有自由基(如羟自由基、氧自由基)清除活性的类黄酮抑制了拟南芥干旱胁迫下的活性氧产生,提高了拟南芥的抗旱性[27]。在失水胁迫期间,坛紫菜中的黄酮类物质的含量升高,暗示着黄酮类物质也参与对失水胁迫的响应。
在失水胁迫期间上调的坛紫菜代谢物中还有一些相容性溶质,如脯氨酸、亮氨酸、甜菜碱等。以往有研究在各种耐旱植物中检测到脯氨酸水平的增加[28-29]。转录水平上脯氨酸代谢的显著变化证明脯氨酸积累是植物的应激诱导和适应性反应[30]。在失水胁迫条件下,脯氨酸积累可能通过降低细胞质渗透势[31-32]以及充当水的替代品来保护细胞结构[33]。甜菜碱是常见的两性离子季铵盐化合物之一,在干旱条件下可作为渗透保护剂[34]。因此这类物质在坛紫菜失水胁迫期间上调,原因可能是作为渗透压调节剂,来维持细胞膨胀并稳定细胞蛋白质和结构。
干旱胁迫会导致植物脂质含量减少,比如引起细胞膜降解[35-36]、抑制脂质生物合成[37-38]、导致脂肪的分解[39]及过氧化[40]。植物可能通过一些机制重新排列膜脂[41-42]来维持膜结构和流动性,抵抗胁迫损害。在坛紫菜失水胁迫期间,多种甘油磷脂成分发生上调,其中大多含有不饱和脂肪酸,这说明坛紫菜可以通过积极合成膜脂来响应胁迫,增强失水胁迫期间细胞膜的流动性,降低损伤。与失水时相比,在复水阶段,坛紫菜中甘油磷脂的含量回落,基本恢复至对照组水平,说明复水阶段坛紫菜生理得到恢复,并且再次通过改变膜脂成分含量来调整膜的流动性,维持正常生理。
值得注意的是,在此次代谢组非靶向检测中,发现了植物激素脱落酸、反式玉米素及水杨酰咖啡酸在坛紫菜失水-复水过程中的显著变化,说明植物激素可能参与坛紫菜的失水胁迫。植物中不同组织器官中的ABA浓度增加可以促进气孔关闭,减少蒸腾失水,与此同时加强根部吸水,提高植物抗旱力[43-44]。不过,与植物在响应干旱胁迫时会积累ABA不同,坛紫菜中的ABA含量在失水胁迫后有所降低,这暗示坛紫菜这种潮间带藻类具有独特的不依赖于ABA的抗失水胁迫机制。后续研究可以采取靶向检测方式测定坛紫菜失水胁迫过程中植物激素的变化,以探究植物激素响应坛紫菜失水胁迫的机制。
本研究结果对认识坛紫菜的代谢组、研究其重要的代谢通路、寻找坛紫菜失水过程中的标记性代谢物提供了重要的基础数据。后期可以结合转录组学、生理学等其他实验进一步互相验证和研究坛紫菜失水胁迫响应机制。
| [1] |
Jin C, Wang J, Wang S, et al. Porphyra Species: A mini-review of its pharmacological and nutritional properties[J]. Journal of Medicinal Food, 2016, 19(2): 111-119. DOI:10.1089/jmf.2015.3426 (  0) 0) |
| [2] |
张学成. 海藻遗传学[M]. 北京: 中国农业出版社, 2005. Zhang X C. Algal Genetics[M]. Beijing: Agricultaral Press, 2005. (  0) 0) |
| [3] |
Blouin N A, Brodie J A, Grossman A C, et al. Porphyra: A marine crop shaped by stress[J]. Trends in Plant Science, 2011, 16(1): 29-37. DOI:10.1016/j.tplants.2010.10.004 (  0) 0) |
| [4] |
Cock J M, Coelho S M. Algal models in plant biology[J]. Journal of Experimental Botany, 2011, 62(8): 24-25. (  0) 0) |
| [5] |
López-Cristoffanini C, Zapata J, Gaillard F, et al. Identification of proteins involved in desiccation tolerance in the red seaweed Pyropia orbicularis (Rhodophyta, Bangiales)[J]. Proteomics, 2016(15): 23-24. (  0) 0) |
| [6] |
Dettmer K, Aronov P A, Hammock B D. Mass spectrometry-based metabolomics[J]. Mass Spectrometry Reviews, 2010, 26(1): 51-78. (  0) 0) |
| [7] |
Sanchez D H, Redestig H, Kr Mer U, et al. Metabolome-ionome-biomass interactions: What can we learn about salt stress by multiparallel phenotyping?[J]. Plant Signaling & Behavior, 2008, 3(8): 598-600. (  0) 0) |
| [8] |
Atsushi F, Miyako K. A network perspective on nitrogen metabolism from model to crop plants using integrated 'omics' approaches[J]. Journal of Experimental Botany, 2014(19): 5619-5630. (  0) 0) |
| [9] |
Shulaev V, Cortes D, Miller G, et al. Metabolomics for plant stress response[J]. Physiologia Plantarum, 2010, 132(2): 199-208. (  0) 0) |
| [10] |
Roessner U. Drought responses of leaf tissues from wheat cultivars of differing drought tolerance at the metabolite level[J]. Molecular Plant, 2012, 5(2): 418-429. DOI:10.1093/mp/ssr114 (  0) 0) |
| [11] |
Cramer G R, A Ergül, Grimplet J, et al. Water and salinity stress in grapevines: Early and late changes in transcript and metabolite profiles[J]. Functional & Integrative Genomics, 2007, 7(2): 111-134. (  0) 0) |
| [12] |
Foito A, Byrne S L, Shepherd T, et al. Transcriptional and metabolic profiles of Lolium perenne L. genotypes in response to a PEG-induced water stress[J]. Plant Biotechnology Journal, 2010, 7(8): 719-732. (  0) 0) |
| [13] |
Levi A, Paterson A H, Ca Kmak I, et al. Metabolite and mineral analyses of cotton near-isogenic lines introgressed with QTLs for productivity and drought-related traits[J]. Physiologia Plantarum, 2011, 141(3): 265-275. DOI:10.1111/j.1399-3054.2010.01438.x (  0) 0) |
| [14] |
Mane S P, Robinet C V, Ulanov A, et al. Molecular and physiological adaptation to prolonged drought stress in the leaves of two Andean potato genotypes[J]. Functional Plant Biology, 2008, 35(8): 669-688. DOI:10.1071/FP07293 (  0) 0) |
| [15] |
Cecilia V R, Shrinivasrao P M, Alexander V U, et al. Physiological and molecular adaptations to drought in Andean potato genotypes[J]. Journal of Experimental Botany, 2008, 59(8): 2109-2123. DOI:10.1093/jxb/ern073 (  0) 0) |
| [16] |
Rizhsky L. When defense pathways collide. the response of arabidopsis to a combination of drought and heat stress[J]. Plant Physiology, 2004, 134(4): 1683-1696. DOI:10.1104/pp.103.033431 (  0) 0) |
| [17] |
Sanchez D H, Schwabe F, Erban A, et al. Comparative metabolomics of drought acclimation in model and forage legumes[J]. Plant, Cell & Environment, 2011, 35(1): 136-149. (  0) 0) |
| [18] |
Silvente S, Sobolev A P, Lara M. Metabolite adjustments in drought tolerant and sensitive soybean genotypes in response to water stress[J]. PLoS One, 2012, 7(6): e38554. DOI:10.1371/journal.pone.0038554 (  0) 0) |
| [19] |
Semel Y, Schauer N, Roessner U, et al. Metabolite analysis for the comparison of irrigated and non-irrigated field grown tomato of varying genotype[J]. Metabolomics, 2007, 3(3): 289-295. DOI:10.1007/s11306-007-0055-5 (  0) 0) |
| [20] |
王莉. 坛紫菜响应失水胁迫的转录组和表达谱特征分析[D]. 青岛: 中国海洋大学, 2013. Wang L. Analycis of Transcriptome and Expression Profiles in Response to Clesiccation Stress on Pyropia haitanensis[D]. Qingdao: Ocean University of China, 2013. (  0) 0) |
| [21] |
Sun P P, Tang X H, Bi G Q, et al. Gene expression profiles of Pyropia yezoensis in response to dehydration and rehydration stresses[J]. Marine Genomics, 2018(43): 43-49. (  0) 0) |
| [22] |
Wang D, You W, Chen N, et al. Comparative quantitative proteomics reveals the desiccation stress responses of the intertidal seaweed Pyropia haitanensis[J]. Journal of Phycology, 2020, 56(6): 1664-1675. DOI:10.1111/jpy.13052 (  0) 0) |
| [23] |
Cao M, Xu K, Yu X, et al. A chromosome-level genome assembly of Pyropia haitanensis (Bangiales, Rhodophyta)[J]. Mol Ecol Resour, 2020, 20(1): 216-227. DOI:10.1111/1755-0998.13102 (  0) 0) |
| [24] |
Starr R C, Zeikus J A. The culture collection of algae at the University of Texas at Austin[J]. Journal of Phycology, 2010, 29(s4): 1-106. (  0) 0) |
| [25] |
Minoru K, Susumu G, Shuichi K, et al. The KEGG resource for deciphering the genome[J]. Nucleic Acids Res, 2004, 1(32): 277-280. (  0) 0) |
| [26] |
Ma D, Sun D, Wang C, et al. Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress[J]. Plant Physiol Biochem, 2014(80): 60-66. (  0) 0) |
| [27] |
Nakabayashi R, Yonekura-Sakakibara K, Urano K, et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids[J]. Plant Journal, 2014, 77(3): 367-379. DOI:10.1111/tpj.12388 (  0) 0) |
| [28] |
Parida A K, Dagaonkar V S, Phalak M S, et al. Differential responses of the enzymes involved in proline biosynthesis and degradation in drought tolerant and sensitive cotton genotypes during drought stress and recovery[J]. Acta Physiologiae Plantarum, 2008, 30(5): 619-627. DOI:10.1007/s11738-008-0157-3 (  0) 0) |
| [29] |
Evers D, I Lefèvre, Legay S, et al. Identification of drought-responsive compounds in potato through a combined transcriptomic and targeted metabolite approach[J]. Journal of Experimental Botany, 2010, 61(9): 2327-2343. DOI:10.1093/jxb/erq060 (  0) 0) |
| [30] |
Verslues P E, Sharma S. Proline. metabolism and its implications for plant-environment interaction[J]. Arabidopsis Book, 2010, 8(e0140): e0140. (  0) 0) |
| [31] |
Voetberg G S, Sharp R E. Growth of the maize primary root at low water potentials: Ⅲ. Role of increased proline deposition in osmotic adjustment[J]. Plant Physiology, 1991, 96(4): 1125-1130. DOI:10.1104/pp.96.4.1125 (  0) 0) |
| [32] |
Ober E S, Sharp R E. Proline accumulation in maize (Zea mays L.) primary roots at low water potentials (Ⅰ. Requirement for increased levels of abscisic acid)[J]. Plant Physiology, 1994, 105(3): 981-987. DOI:10.1104/pp.105.3.981 (  0) 0) |
| [33] |
Yancey P H. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses[J]. Journal of Experimental Biology, 2005, 208(15): 2819-2830. DOI:10.1242/jeb.01730 (  0) 0) |
| [34] |
Hanson A D, Rathinasabapathi B, Rivoal J, et al. Osmoprotective compounds in the Plumbaginaceae: A natural experiment in metabolic engineering of stress tolerance[J]. Proceedings of the National Academy of Sciences of the United States of America, 1994, 91(1): 306-310. DOI:10.1073/pnas.91.1.306 (  0) 0) |
| [35] |
Anh T, Borrelflood C, Jorge V, et al. Effects of water stress on lipid metabolism in cotton leaves[J]. Phytochemistry, 1985, 24(4): 723-727. DOI:10.1016/S0031-9422(00)84884-0 (  0) 0) |
| [36] |
Paula F M D, Thi A, Silva J, et al. Effects of water stress on the molecular species composition of polar lipids from Vigna unguiculata L. leaves[J]. Plant Science, 1990, 66(2): 185-193. DOI:10.1016/0168-9452(90)90203-Z (  0) 0) |
| [37] |
Thi A, Borrel-Flood C, Silva J, et al. Effects of drought on[1-14C]-oleic and[1-14C]-linoleic acid desaturation in cotton leaves[J]. Physiologia Plantarum, 1987, 69(1): 147-150. DOI:10.1111/j.1399-3054.1987.tb01958.x (  0) 0) |
| [38] |
Paula F M D, Thi A T P, Zuily-Fodil Y, et al. Effects of water stress on the biosynthesis and degradation of polyunsaturated lipid molecular species in leaves of Vigna unguiculata[J]. Plant Physiology & Biochemistry, 1993, 31(5): 707-715. (  0) 0) |
| [39] |
Ferrari-Iliou Roselyne, D'arcy-Lameta Agnès, Thu Pham Thi Anh. Effect of drought on photodynamic peroxidation of leaf total lipophilic extracts[J]. Pergamon, 1994, 37(5): 1237-1243. (  0) 0) |
| [40] |
Sahsah Y, Campos P, Gareil M, et al. Enzymatic degradation of polar lipids in Vigna unguiculata leaves and influence of drought stress[J]. Physiologia Plantarum, 1998, 104(4): 577-586. DOI:10.1034/j.1399-3054.1998.1040409.x (  0) 0) |
| [41] |
Lösch R. Plant water relations[M]//Ellenberg H, Esser K, Kubitzki K, et al. Progress in Botany. New York: Springer, 1989: 27-50.
(  0) 0) |
| [42] |
Turner N C, Jones M M. Turgor maintenance by osmotic adjustment: A review and evaluation[J]. Adaptation of Plants to Water & High Temperature Stress, 1980, 1: 87-103. (  0) 0) |
| [43] |
Schroeder J I, Kwak J M, Allen G J. Guard cell abscisic acid signalling and engineering drought hardiness in plants[J]. Nature, 2001, 410(6826): 327-330. DOI:10.1038/35066500 (  0) 0) |
| [44] |
Verslues P E, Zhu J K. Before and beyond ABA: Upstream sensing and internal signals that determine ABA accumulation and response under abiotic stress[J]. Biochemical Society Transactions, 2005, 33(2): 375-379. DOI:10.1042/BST0330375 (  0) 0) |
2. School of Marine Science and Engineering, Qingdao Agricultural University, Qingdao 266237, China;
3. College of Fisheries and Life Science, Hainan Tropical Ocean University, Sanya 572022, China
 2022, Vol. 52
2022, Vol. 52


