细菌细胞膜表面含有大量的寡糖链,这些寡糖链在细菌与宿主细胞之间的识别中发挥了关键的作用。6-脱氧七碳糖作为革兰氏阴性菌表面糖链的重要组成单元,在免疫识别方面发挥重要的作用[1-2]。目前为止,已从天然界中发现了六种6-脱氧七碳糖的存在,分别是:6-脱氧-D-甘露七碳糖,6-脱氧-D-艾杜七碳糖,6-脱氧-D-塔洛七碳糖,6-脱氧-L-半乳七碳糖,6-脱氧-D-阿卓七碳糖和6-脱氧-L-古洛七碳糖[2]。研究表明,该类化合物在自然界存在稀少,无法通过分离提取获得足够量以用于实验研究,因此发展一种简洁高效的方法对其进行制备具有重要的意义。
目前,七碳糖的合成方法主要有:以六碳糖为原料,通过亲核取代反应[3],Wittig反应[4]实现碳链的增长;以五碳糖为原料,通过亲核加成反应实现碳链的延伸[5]。本文旨在发展一种采用“首尾翻转”策略,通过脱羧氟代反应合成正交保护的6-脱氧古洛七碳糖氟苷的方法,为制备6-脱氧七碳糖提供了新思路。
1 实验部分 1.1 主要实验仪器及试剂Agilent DD2 (500 MHz)核磁共振波谱仪;质谱仪(Q-Tof Global);旋转蒸发仪(BUCHI, DTC-21,R-114:EYELA);Selectfluor®为安耐吉分析纯试剂,碳酸银(Ag2CO3)为阿拉丁分析纯试剂,N, N-二甲基甲酰胺(DMF),4-N, N-二甲基吡啶(DMAP),对溴苄溴(PBBBr),溴化苄(BnBr),叔丁基二苯基氯硅烷(TBDPSCl),苯甲酰氯(BzCl),四丁基氟化铵(TBAF),二醋酸碘苯(BAIB),锇酸钾(K2OsO4),高碘酸钠(NaIO4),硼氢化钠(NaBH4),对甲苯磺酸(p-TsOH),2, 2, 6, 6-四-甲基哌啶氮氧化物(TEMPO),二水合氟化钾(KF·2H2O)均为国产分析纯试剂;吡啶用4Å分子筛干燥,丙酮用高锰酸钾蒸馏。
1.2 化合物的合成 1.2.1 化合物24, 6-O-苄基-1-脱氧-1-C-烯丙基-(-D-半乳糖苷1 (213 mg, 0.73 mmol, 1 equiv)溶于DMF (4 mL)中,冰浴冷却下,加入NaH (87 mg, 2.19 mmol, 3.0 equiv)和PBBBr (460 mg, 1.83 mmol, 2.5 equiv)。将反应液温度逐渐升至室温并在此温度下搅拌6 h。反应完全后, 加入甲醇淬灭反应。减压除去DMF后,剩余残余物溶于二氯甲烷中,依次用水和饱和食盐水洗涤,合并有机相。有机相用无水硫酸钠干燥,抽滤,浓缩后,残余物经硅胶柱层析(石油醚:乙酸乙酯=10:1)得白色固体化合物2 (430 mg, 0.68 mmol, 94%)。[α]D27 = +83.58 (c 0.80, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.56-7.49 (m, 2H), 7.48-7.40 (m, 4H), 7.40-7.32 (m, 3H), 7.26-7.15 (m, 4H), 5.88-5.78 (m, 1H, H-9), 5.49 (s, 1H, H-7), 5.14-5.05 (m, 2H), 4.74-4.63 (m, 3H), 4.59 (d, J = 11.8 Hz, 1H), 4.36-4.30 (m, 1H, H-1), 4.26-4.24 (m, 1H), 4.23-4.17 (m, 2H), 4.00 (dd, J = 12.4, 1.9 Hz, 1H, H-2), 3.73 (dd, J = 9.9, 3.6 Hz, 1H, H-3), 3.46 (d, J = 1.7 Hz, 1H, H-4), 2.52-2.44 (m, 1H, H-1′), 2.42-2.34 (m, 1H, H-1′); 13C NMR (126 MHz, CDCl3) δ 137.9, 137.9, 137.8, 137.5, 135.0, 131.6, 129.3, 129.1, 128.3, 126.5, 121.2 (C-2′), 116.7 (C-3′), 101.3 (C-1), 77.2, 76.8, 75.8, 74.6, 72.9, 72.9, 70.8, 70.1, 29.6 (C-1′); HRMS (ESI) m/z Calcd for C30-H31O5Br2 [M+H]+ 629.053 3, found 629.052 5。
1.2.2 化合物3和3′化合物2 (357 mg, 5.67 mmol, 1 equiv)溶于四氢呋喃(15 mL)中,加入活化的4Å分子筛(1.5 g),反应液在室温下搅拌15 min。将反应液冷却至0 ℃,加入碘(290 mg, 11.34 mmol, 2 equiv)。搅拌3 h后,将分子筛过滤,反应液用饱和硫代硫酸钠水溶液淬灭,二氯甲烷和水稀释,萃取,合并收集有机相。有机相用饱和食盐水洗涤,无水硫酸钠干燥,抽滤,浓缩后,残余物经硅胶柱层析(石油醚:乙酸乙酯=8:1)得淡黄色浆状化合物3和3′ (309 mg, 5.27 mmol, 3/3′=1:1.5, 93%)。化合物3: [α]D27 = +5.31 (c 1.86, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.52-7.41 (m, 4H), 7.40-7.31 (m, 5H), 5.49 (s, 1H), 5.16-5.06 (m, 1H, H-1), 4.80 (d, J = 12.6 Hz, 1H), 4.70 (d, J = 12.6 Hz, 1H), 4.41-4.37 (m, 1H), 4.34 (dd, J = 12.7, 1.3 Hz, 1H), 4.23 (dd, J = 5.4, 4.4 Hz, 1H), 4.17-4.10 (m, 1H, H-2′), 3.63 (dd, J = 5.4, 2.0 Hz, 1H), 3.58-3.56 (m, 1H), 3.31-3.29 (m, 2H, H-3′), 2.40-2.34 (m, 1H, H-1′), 1.91-1.86 (m, 1H, H-1′); 13C NMR (126 MHz, CDCl3) δ 137.9, 137.1, 131.8, 130.1, 129.3, 128.4, 126.4, 121.8, 100.8 (C-1), 84.3, 80.0, 78.4, 77.8, 77.2, 73.6, 70.8, 67.6, 61.8, 38.6 (C-3′), 9.9 (C-1′); HRMS (ESI) m/z Calcd for C23 H25O5BrI [M+H]+ 586.9925, found 586.9917.化合物3′: [α]D27 = -5.37 (c 0.60, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.47-7.43 (m, 4H), 7.41-7.29 (m, 5H), 5.48 (s, 1H), 5.21 (t, J = 4.7 Hz, 1H, H-1), 4.80 (d, J = 12.6 Hz, 1H), 4.68 (d, J = 12.7 Hz, 1H, H-2), 4.45 (dd, J = 5.3, 3.9 Hz, 1H), 4.40 (t, J = 2.6 Hz, 1H), 4.36-4.29 (m, 1H), 4.25-4.20 (m, 1H, H-2′), 4.06 (dd, J = 12.8, 2.2 Hz, 1H), 3.59-3.56 (m, 2H), 3.33-3.25 (m, 2H, H-3′), 2.24-2.20 (m, 1H, H-1′), 1.84-1.78 (m, 1H, H-1′); 13C NMR (126 MHz, CDCl3) δ 137.9, 137.3, 131.7, 129.9, 129.2, 128.4, 126.4, 121.7, 100.4 (C-1), 84.1, 79.6, 78.3, 78.0, 77.2, 73.7, 72.8, 70.2, 67.6, 40.0 (C-3′), 10.2 (C-1′); HRMS (ESI) m/z Calcd for C23H25O5BrI [M+H]+ 586.992 5, found 586.991 4。
1.2.3 化合物4化合物3和3′的混合物(720 mg, 1.22 mmol, 1 equiv)溶于四氢呋喃/水(v/v=2:1, 15 mL),加入锌粉(800 mg, 12.26 mmol, 10 equiv),混合物在50 ℃下反应过夜。将锌粉过滤,二氯甲烷和水稀释,分离和收集有机相。有机相用饱和食盐水洗涤,无水硫酸钠干燥,抽滤,减压浓缩后,残余物经硅胶柱层析(石油醚:乙酸乙酯=3:1)得白色固体化合物4 (512 mg, 1.11 mmol, 91%)。[α]D27 = +106.75 (c 0.60, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.55-7.49 (m, 2H), 7.49-7.43 (m, 2H), 7.41-7.31 (m, 3H), 7.29-7.23 (m, 2H), 5.95-5.75 (m, 1H, H-2′), 5.46 (s, 1H), 5.17-5.05 (m, 2H), 4.70 (d, J = 12.0 Hz, 1H), 4.59 (d, J = 12.0 Hz, 1H), 4.37 (dddd, J = 20.8, 11.1, 6.0, 3.0 Hz, 2H), 4.28-4.18 (m, 2H), 4.01 (dd, J = 12.4, 1.9 Hz, 1H), 3.63 (dd, J = 9.6, 3.5 Hz, 1H), 3.49 (q, J = 1.6 Hz, 1H), 2.55-2.45 (m, 1H, H-3′), 2.41-2.28 (m, 1H, H-3′); 13C- NMR (126 MHz, CDCl3) δ 135.0, 131.7, 129.6, 129.1, 128.3, 126.4 (C-2′), 116.9 (C-3′), 101.1 (C-1), 77.1, 75.5, 73.1, 70.2, 70.1, 67.4, 63.2, 29.0 (C-1′); HRMS (ESI) m/z Calcd for C23H26-O5Br [M+H]+ 461.095 8, found 461.095 5。
1.2.4 化合物5按照合成化合物2的操作步骤,化合物4 (1.20 g, 2.65 mmol, 1 equiv)与BnBr (0.47 mL, 3.98 mmol, 1.5 equiv)在DMF(15 mL)中反应1 h。后处理后所得残余物经硅胶柱层析(石油醚:乙酸乙酯=6:1)得白色固体化合物5 (1.33 g, 2.41 mmol, 91%)。[α]D27 = +90.27 (c 0.37, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.54-7.52 (m, 2H), 7.45-7.42 (m, 2H), 7.39-7.33 (m, 7H), 7.31-7.25 (m, 3H), 5.87-5.79 (m, 1H, H-2′), 5.50 (s, 1H, H-7), 5.15-5.04 (m, 2H, H-3′), 4.78 (dd, J = 11.5, 1.9 Hz, 1H), 4.71 (s, 2H), 4.65 (dd, J = 11.7, 1.9 Hz, 1H), 4.37-4.30 (m, 1H, H-1), 4.26-4.18 (m, 3H), 4.01 (d, J=12.3 Hz, 1H, H-6), 3.75-3.72 (m, 1H, H-3), 3.46 (s, 1H, H-4), 2.53-2.49 (m, 1H, H-1′), 2.41-2.34 (m, 1H, H-1′); 13C NMR (126 MHz, CDCl3) δ 138.6, 137.9, 137.7, 135.1, 129.3, 129.0, 128.4, 128.2, 127.7, 127.7, 121.5 (C-2′), 116.7 (C-3′), 110.1, 101.3 (C-1), 76.7, 75.7, 74.6, 73.6, 70.9, 70.8, 70.0, 63.1, 29.5 (C-1′); HRMS (ESI) m/z Calcd for C30H32O5Br [M+H]+ 551.142 8, found 551.142 5。
1.2.5 化合物6化合物5 (1.40 g, 2.54 mmol, 1 equiv)溶于四氢呋喃/水(v/v=3:1, 15 mL)中,依次加入K2OsO4 (187 mg, 0.51 mmol, 0.2 equiv)和NaIO4 (2.17 g, 10.16 mmol, 4 equiv),室温下反应过夜。反应液用饱和硫代硫酸钠水溶液淬灭,二氯甲烷和水稀释,分离和收集有机相。有机相用饱和食盐水洗涤,无水硫酸钠干燥,抽滤,减压浓缩后,将所得粗产品溶于四氢呋喃(14 mL)中,0 ℃下加入NaBH4 (96 mg, 2.54 mmol, 1 equiv),1 h后结束。反应液用水淬灭。二氯甲烷和水稀释,分离和收集有机相。有机相用饱和食盐水洗涤,无水硫酸钠干燥,抽滤,减压浓缩后,残余物经硅胶柱层析(石油醚:乙酸乙酯= 3:1)得白色固体化合物6 (1.07 g, 1.93 mmol, 两步76%)。[α]D27=+58.07 (c 0.57, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.55-7.49 (m, 2H), 7.46-7.41 (m, 2H), 7.41-7.27 (m, 7H), 7.28-7.23 (m, 2H), 5.50 (s, 1H, H-7), 4.80 (d, J = 11.5 Hz, 1H), 4.70 (s, 2H), 4.64 (d, J = 11.5 Hz, 1H), 4.43-4.38 (m, 1H, H-1), 4.27-4.21 (m, 2H), 4.19 (dd, J = 12.5, 1.6 Hz, 1H, H-6), 4.02 (dd, J = 12.5, 1.8 Hz, 1H, H-6), 3.77 (t, J=6.1 Hz, 2H, H-2′), 3.73 (dd, J=9.9, 3.5 Hz, 1H), 3.55 (d, J = 1.8 Hz, 1H, H-4), 2.01-1.85 (m, 2H, H-1′); 13C NMR (126 MHz, CDCl3) δ 138.4, 138.4, 137.8, 137.7, 137.6, 137.6, 131.5, 129.3, 128.5, 128.3, 127.9, 127.8, 126.4, 121.3, 101.3 (C-1), 76.8, 75.3, 74.7, 74.5, 73.9, 70.9, 70.0, 63.7, 61.4 (C-2′), 27.6 (C-1′); HRMS (ESI) m/z Calcd for C29H32O6Br [M+H]+ 555.137 7, found 555.137 6。
1.2.6 化合物7按照合成化合物2的操作步骤,化合物6 (5.60 g, 10.11 mmol, 1 equiv)与BnBr (1.80 mL, 15.17 mmol, 1.5 equiv)在DMF (30 mL)中反应1 h。后处理后所得残余物经硅胶柱层析(石油醚:乙酸乙酯=5:1)得白色固体化合物7 (5.86 g, 9.09 mmol, 90%)。[α]D18 = +53.09 (c 1.37, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.54-7.49 (m, 2H), 7.43-7.39 (m, 2H), 7.38-7.21 (m, 14H), 5.48 (s, 1H, H-7), 4.74 (d, J = 11.6 Hz, 1H), 4.68 (s, 2H), 4.62 (d, J = 11.5 Hz, 1H), 4.50 (s, 1H), 4.49 (s, 1H), 4.44-4.38 (m, 1H, H-1), 4.24-4.18(m, 2H), 4.15(dd, J=12.4, 1.6 Hz, 1H), 3.97 (dd, J = 12.3, 1.8 Hz, 1H), 3.69 (dd, J = 9.9, 3.5 Hz, 1H), 3.58-3.52 (m, 2H, H-2′), 3.44 (d, J = 1.5 Hz, 1H, H-4), 2.12-2.05 (m, 1H, H-1′), 1.89-1.82 (m, 1H, H-1′); 13C- NMR (126 MHz, CDCl3) δ 138.7, 138.6, 137.8, 137.7, 131.5, 129.4, 129.0, 128.5, 128.4, 128.2, 127.7, 127.7, 127.7, 127.6, 126.5, 121.5, 101.3 (C-1), 76.7, 75.6, 74.7, 73.5, 73.3, 72.5, 70.9, 70.1, 67.5, 63.3 (C-2′), 25.2 (C-1′); HRMS (ESI) m/z Calcd for C36H41O6N81Br [M+NH4]+ 664.209 1, found 664.209 6。
1.2.7 化合物8化合物7 (5.86 g, 9.09 mmol, 1 equiv)溶于二氯甲烷/甲醇(v/v=1:1, 60 mL)中,加入p-TsOH (313 mg, 1.82 mmol, 2 equiv),反应于40 ℃下过夜。反应液用三乙胺淬灭,减压除去溶剂后,剩余残余物溶于二氯甲烷中,依次用水和饱和食盐水洗涤,合并有机相。有机相用无水硫酸钠干燥,抽滤,浓缩后,残余物经硅胶柱层析(石油醚:乙酸乙酯= 1:1)得白色固体化合物8 (4.80 g, 8.64 mmol, 95%)。[α]D18 = +25.72 (c 0.95, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.45 (d, J = 8.3 Hz, 2H), 7.37-7.27 (m, 9H), 7.19 (d, J = 8.1 Hz, 2H), 4.68-4.60 (m, 4H), 4.54 (d, J = 12.0 Hz, 1H), 4.48 (d, J = 12.0 Hz, 1H), 4.35-4.30 (m, 1H, H-1), 4.06-4.04 (m, 1H), 3.96 (dd, J = 9.0, 5.7 Hz, 1H), 3.88 (dd, J = 11.6, 6.8 Hz, 1H), 3.71-3.66 (m, 1H), 3.64-3.58 (m, 2H), 3.58-3.52 (m, 2H), 2.57 (s, 1H, OH), 2.14-2.12 (s, 1H, OH), 2.03-1.96 (m, 1H, H-1′), 1.95-1.84 (m, 1H, H-1′); 13C NMR (126 MHz, CDCl3) δ 138.4, 136.9, 131.7, 129.4, 128.5, 128.5, 127.9, 127.8, 127.8, 127.8, 75.6, 73.2, 73.1, 71.8, 70.9, 70.7, 68.4, 66.7, 62.7 (C-2′), 25.4 (C-1′); HRMS (ESI) m/z Calcd for C29H37O6NBr [M+NH4]+ 574.179 9, found 574.179 7。
1.2.8 化合物9化合物8 (5.80 g, 10.37 mmol, 1 equiv)溶于DMF (52 mL)中,依次加入TBDPSCl (3.24 mL, 12.44 mmol, 1.2 equiv)和咪唑(1.43 g, 20.74 mmol, 2 equiv),室温下过夜反应。将DMF除去,残余物溶于二氯甲烷中,依次用水和饱和食盐水洗涤,合并有机相。有机相用无水硫酸钠干燥,抽滤,减压浓缩后将所得残余物溶于吡啶(52 mL)中,冰浴冷却下,向溶液中缓慢加入BzCl (2.38 mL, 20.74 mmol, 2 equiv)和DMAP (633 mg, 5.19 mmol, 0.5 equiv),室温下反应6 h。减压浓缩,残余物用二氯甲烷溶解,依次用1 mol·L-1的盐酸溶液和饱和碳酸氢钠溶液洗涤,合并并收集有机相。有机相用无水硫酸钠干燥,过滤,浓缩后残余物经硅胶柱层析(石油醚:乙酸乙酯=12:1)得粗产品。将粗产品溶于四氢呋喃(52 mL)中,冰浴冷却下,依次加入AcOH (1.45 mL, 25.93 mmol, 2.5 equiv)和TBAF (5.69 mL, 20.74 mmol, 2 equiv),室温下搅拌过夜。反应液用二氯甲烷和水稀释,分离,收集有机相。有机相用饱和食盐水洗涤,无水硫酸钠干燥,抽滤,浓缩后残余物经硅胶柱层析(石油醚:乙酸乙酯=3:1)得粗产品。称取上一步粗产品(4.18 g, 6.33 mmol, 1 equiv)溶于二氯甲烷/水(v/v = 3:1, 60 mL)中,依次加入BAIB (5.10 g, 15.84 mmol, 2.5 equiv)和TEMPO (300 mg, 1.9 mmol, 0.3 equiv),室温下反应过夜。反应液用饱和硫代硫酸钠水溶液淬灭,二氯甲烷萃取,饱和食盐水洗涤,合并有机相,有机相用无水硫酸钠干燥,过滤,浓缩后,残余物经硅胶柱层析(石油醚:乙酸乙酯=1:1)得白色固体化合物9 (3.58 g, 5.31 mmol, 四步71%)。[α]D14 = +50.02 (c 0.50, CHCl3); 1H NMR (500 MHz, CDCl3) δ 8.00 (d, J = 8.3 Hz, 2H), 7.59-7.53 (m, 1H), 7.41 (t, J = 7.7 Hz, 2H), 7.39-7.35 (m, 2H), 7.35-7.27 (m, 7H), 7.26-7.22 (m, 2H), 7.16-7.11 (m, 2H), 6.01 (dd, J = 4.0, 2.8 Hz, 1H, H-5), 4.77 (d, J = 11.7 Hz, 1H), 4.68 (d, J = 11.7 Hz, 1H), 4.60 (d, J = 4.1 Hz, 1H), 4.56-4.46 (m, 5H), 3.90 (dd, J = 7.0, 2.8 Hz, 1H), 3.76 (dd, J = 7.0, 4.2 Hz, 1H), 3.70-3.58 (m, 2H), 2.07-1.98 (m, 1H), 1.98-1.86 (m, 1H); 13C NMR (126 MHz, CDCl3) δ 170.2 (COOH), 165.5 (COPh), 136.7, 133.4, 131.6, 129.9, 129.7, 128.6, 128.6, 128.5, 128.0, 128.0, 128.0, 127.9, 77.1, 76.1, 73.5, 73.3, 72.0, 71.6, 70.9, 68.3, 66.9, 27.4; HRMS (ESI)m/z Calcd for C36H34O8Br[M+H]+673.144 3。
1.2.9 化合物10化合物9 (201 mg, 0.29 mmol, 1 equiv)溶于丙酮/水(v/v=6:1, 10.5 mL)中,氩气保护下,依次加入Selectfluor®(528 mg, 1.49 mmol, 5.14 equiv),Ag2CO3 (41 mg, 0.15 mmol, 0.5 equiv)以及KF2H2O (140 mg, 1.49 mmol, 5.13 equiv),反应体系重复抽真空—氩气置换步骤3次,反应液在25 ℃下反应1 h,过滤除去银盐。将滤液倾入水中,水相用二氯甲烷萃取。合并并用无水硫酸钠干燥有机相,过滤。浓缩滤液后残余物经硅胶柱层析(石油醚:乙酸乙酯= 10:1),得淡黄色浆状物化合物10 (86 mg, 0.13 mmol, α/β= 1:1.2, 46%)。β: [α]D27 = -3.60 (c 1.65, CHCl3); 1H NMR (500 MHz, CDCl3) δ 8.03 (d, J = 7.7 Hz, 2H), 7.70-7.25 (m, 16H), 7.01 (d, J = 7.9 Hz, 2H), 5.69 (dd, J = 6.9 Hz, JH-F = 54.2 Hz, 1H), 5.34-5.30 (m, 1H), 4.67 (d, J = 12.0 Hz, 1H), 4.55-4.45 (m, 3H), 4.42-4.38 (m, 2H), 4.34-4.31 (m, 1H), 4.17 (d, J =4.6 Hz, 1H), 3.63-3.59 (m, 1H), 3.55-3.44 (m, 2H), 2.15-2.08 (m, 1H), 1.91-1.84 (m, 1H); 13C NMR (126 MHz, CDCl3) δ 138.5, 137.4, 136.5, 133.5, 131.6, 129.9, 129.5, 129.5, 128.6, 128.5, 128.4, 128.2, 127.8, 127.8, 105.5 (JC-F= 213.6 Hz), 73.9 (JC-F= 6.8 Hz), 73.2, 72.8, 72.5, 71.8 (JC-F= 2.1 Hz), 71.3, 71.1, 66.5, 29.8; 19F NMR (470 MHz, CDCl3) δ -142.4; HRMS (ESI) m/z Calcd for C35H38O6N81BrF [M+NH4]+ 668.1841, found 668.1840.[α]D27 = +15.60 (c 0.70, CHCl3); 1H NMR (500 MHz, CDCl3) δ 8.05 (d, J = 7.7 Hz, 2H), 7.61 (t, J = 7.5 Hz, 1H), 7.47 (t, J = 7.7 Hz, 2H), 7.38-7.25 (m, 13H), 7.09 (d, J = 8.0 Hz, 2H), 5.73 (dd, J = 3.1 Hz, JH-F = 54.7 Hz, 1H), 5.36 (dt, J = 3.4 Hz, JH-F = 27.5 Hz, 1H), 4.64 (d, J = 11.9 Hz, 1H), 4.58 (dd, J = 8.9, 4.9 Hz, 1H), 4.55-4.45 (m, 5H), 4.07 (t, J = 3.8 Hz, 1H), 3.58-3.50 (m, 3H), 2.10-2.03 (m, 1H), 1.84-1.77 (m, 1H); 13C NMR (126 MHz, CDCl3) δ 137.3, 136.9, 133.6, 131.6, 130.0, 129.4, 128.7, 128.6, 128.5, 128.4, 128.3, 127.8, 127.7, 105.6 (JC-F= 232.7 Hz), 75.6, 73.3, 72.8, 72.6, 72.0, 68.5 (JC-F= 23.0 Hz), 66.7, 66.3, 29.8; 19F NMR (470 MHz, CDCl3) δ -144.8 to -145.0; HRMS (ESI) m/z Calcd for C35H38O6NBrF [M+NH4]+ 666.186 1。
2 结果与讨论作为D-半乳糖的非对映异构体之一,D-古洛糖的2-,3-,4-位羟基的立体构型与半乳糖相反,因此,我们拟由α-半乳糖醛酸碳苷经脱羧氟代反应,经“首尾翻转”,原先的异头位变为5位,而氟原子所在的5位变为异头位,从而完成6-脱氧古洛七碳糖氟苷的制备。如图 1所示,由α-半乳糖烯丙碳苷1[6]出发,经对溴苄基(PBB)保护得化合物2,在I2的作用下与2位氧原子进行分子内环化反应[7-8],以1:1.5的比例得到非对映异构体混合物3和3′。随后,在锌粉还原条件下,还原开环得到烯丙碳苷4[9]。用Bn保护化合物4的2位羟基得到化合物5,化合物2、4和5均可通过重结晶的方式进行纯化,可实现克级规模的制备。K2OsO4/NaIO4氧化断裂C-C双键[10],再经NaBH4还原可将化合物5转化为伯醇6。对化合物的羟基进行Bn保护得到化合物7。随后,在p-TsOH的作用下脱除苄叉保护基得到二醇8。经TBDPS保护伯羟基、Bz保护仲羟基、脱除硅基和BAIB/TEMPO介导的氧化反应,以4步71%的产率实现化合物8到羧酸9的转化[11]。在Ag2CO3和Selectfluor®的共同作用下,9发生氧化自由基脱羧氟代反应,以46%的产率得到6-脱氧-D-古洛七碳糖氟苷10。综上,我们以α-半乳糖烯丙碳苷1为原料,经13步反应,以30%的总收率合成得到6-脱氧古洛七碳糖氟苷10。
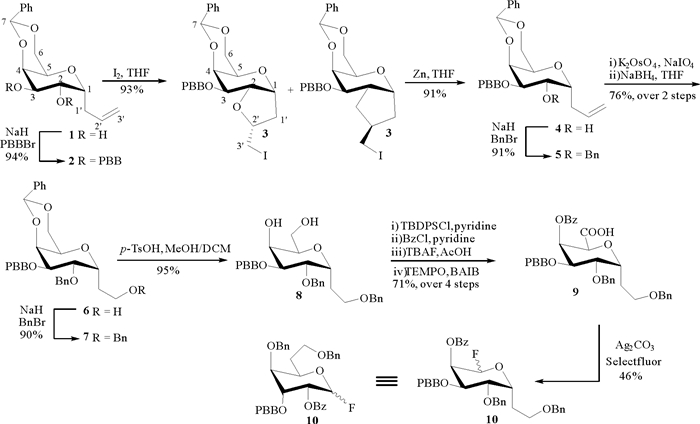
|
图 1 6-脱氧-D-古洛-七碳糖氟苷的合成 Fig. 1 Synthesis of 6-deoxy-D-gulo-heptopyranosyl fluoride |
本文以廉价易得的α-半乳糖烯丙碳苷为原料,以选择性3位PBB保护、糖醛酸脱羧氟代等为关键步骤,以简洁高效的方法合成了正交保护的6-脱氧-D-古洛七碳糖氟苷10,为稀有高碳糖的合成提供了一条新的途径,其中化合物2~10均为首次报道。此外,所制备的高碳糖氟苷可直接作为糖基供体应用于寡糖片段的合成,为合成含有高碳糖的活性天然产物的合成提供了一条新的途径。
| [1] |
Aspinall G O, Mcdonald A G, Sood R K. Syntheses of methyl glycosides of 6-deoxyheptoses[J]. Candian Journal of Chemistry, 1994(72): 247-251.
(  0) 0) |
| [2] |
Pakulski Z, Poly F, Dorabawila N, et al. 6-deoxyheptoses in nature, chemistry, and medicine[J]. Current Organic Chemistry, 2014(18): 1818-1845.
(  0) 0) |
| [3] |
Reckendorf W M Z. Synthesis of 6-deoxy-D-gluco-dialdoheptose and Ring Closure to 8(a), 10(a)-dihydroxy-2, 4, 6-trioxaadamantane[J]. Angewandte Chemie International Edition, 1966(5): 665.
(  0) 0) |
| [4] |
Amigues E J, Greenberg M L, Ju S, et al. Synthesis of cyclophospho-glucoses and glucitols[J]. Tetrahedron, 2007(63): 10042-10053.
(  0) 0) |
| [5] |
Kovensky J, Mallet J M, Esnault J, et al. Further evidence for the critical role of a non-chair conformation of L-iduronic acid in the activation of antithrombin[J]. European Journal of Organic Chemistry, 2002(21): 3595-3603.
(  0) 0) |
| [6] |
Zou W, Shao H W, Wu S H. Intramolecular aldol cyclization of C-4-ulopyranosyl-2′-oxoalkanes controlled by steric effects. Asymmetric synthesis of substituted 8-oxabicyclo [3. 2. 1] octanones and octenones and cyclopentenones[J]. Carbohydrate Research, 2004(339): 2475-2485.
(  0) 0) |
| [7] |
Hager D, Paulitz C, Tiebes J, et al. Total synthesis of herbicidin C and aureonuclemycin: Impasses and new avenues[J]. Journal of Organic Chemistry, 2013(78): 10784-10801.
(  0) 0) |
| [8] |
Cervi G, Peri F, Battistini C, et al. Bicyclic carbohydrate-derived scaffolds for combinatorial libraries[J]. Bioorganic & Medicinal Chemistry, 2006(14): 3349-3367.
(  0) 0) |
| [9] |
Hager D, Mayer P, Paulitz C, et al. Stereoselective total syntheses of herbicidin C and aureonuclemycin through late-stage glycosylation[J]. Angewandte Chemie International Edition, 2012(51): 6525-6528.
(  0) 0) |
| [10] |
Du K, Yang H, Guo P, et al. Efficient syntheses of (-)-crinine and (-)-aspidospermidine, and the formal synthesis of (-)-minfiensine by enantioselective intramolecular dearomative cyclization[J]. Chemical Science, 2017(8): 6247-6256.
(  0) 0) |
| [11] |
Farrell M, Zhou J, Murphy P V. Regiospecific anomerisation of acylated glycosyl azides and benzoylated disaccharides by using TiCl4[J]. Chemistry A European Journal, 2013(19): 14836-14851.
(  0) 0) |
 2020, Vol. 50
2020, Vol. 50


