2. 上海海洋大学水产科学国家级实验教学示范中心,上海 201306
近年来,随着水产养殖业的发展,对鱼粉需求量逐年增加,而全球渔业资源衰退导致鱼粉产量逐年下降,限制了水产饲料中鱼粉的添加量,为了替代鱼粉,国内外进行了大量研究用动植物原料替代鱼粉[1],但蛋白酶抑制剂、凝集素、植酸、皂苷等抗营养因子的存在[2-3],氨基酸的不平衡,脂肪饱和度较高,牛磺酸、羟脯氨酸等促生长因子的缺乏等[4],导致水产动物生长受阻,因此,海洋资源产品的开发成为研究的新浪潮,而鱼溶浆作为鱼粉副产品,成为鱼粉的理想替代品。新鲜海鱼的含水量在70%左右,在鱼粉生产过程中,经过蒸煮、压榨,大量的水溶性物质被分离在压榨液中,经酶解浓缩固化后形成鱼溶浆粉,与植物蛋白源相比,鱼溶浆粉不存在抗营养因子[2],与其它动物蛋白源相比,鱼溶浆保留了鱼粉特有的未知促生长因子和生物活性物质,其特有的鱼腥香味和诱食因子,对水产动物具有很好的诱食和促生长作用,此外,鱼溶浆粉含有植物蛋白源所没有的小分子多肽、核苷酸、牛磺酸以及矿物质等有益成分,可增强动物消化吸收,促进肠道发育,增强免疫机能和抗应激能力,是优质的动物蛋白源、诱食剂和营养补充剂[5-6]。有效利用海洋蛋白源是缓解鱼粉资源短缺和保障水产养殖业可持续发展的重要途径。
珍珠龙胆石斑鱼(Epinephelus fuscoguttatus♀×E. lanceolatu )又称龙虎斑或珍珠斑,隶属于鲈形目(Perciformes)鲈亚目(Percoidei)鮨科(Serranodae)石斑鱼属(Epinephelus),是由鞍带石斑鱼(Epinephelus lanceolatu)为父本,棕点石斑鱼(E. fuscoguttatus)为母本杂交而来,其肉质细嫩、成长快速、抗病力强,集合了虎斑抗病力强和龙胆生长快速的优点,显现杂交优势,极具发展潜力[7-8],是中国海南、福建、广东等地的传统养殖品种。珍珠龙胆石斑鱼是肉食性鱼类,其饲料中鱼粉的添加量较高,目前国内外均未见有珍珠龙胆石斑鱼高植物蛋白饲料中添加鱼溶浆粉的研究报道。本研究通过在高植物蛋白饲料中添加少量鱼溶浆粉,以期达到降低珍珠龙胆石斑鱼饲料鱼粉使用量及降低饲料成本的目的。
1 材料与方法 1.1 实验饲料以白鱼粉、大豆浓缩蛋白为主要蛋白源,鱼油为主要脂肪源,以添加60%的白鱼粉为对照组(DF),各处理组用大豆浓缩蛋白替代30%白鱼粉,并添加0(D0)、2.5%(D2.5)、5%(D5)、7.5%(D7.5)及10%(D10)的鱼溶浆粉(购于荣成市海圣饲料有限公司,营养成分见表 1),用酪蛋白调节蛋白平衡,鱼油调节脂肪平衡,配制成6组等氮等能的实验饲料。所有原料粉碎后过80目,逐级混匀后,加入新鲜鱼油和蒸馏水再次混匀,经螺旋挤压机加工成直径为3与5 mm左右两种规格的饲料颗粒,60 ℃烘干并保存于-20 ℃冰箱备用。饲料配方及营养组成见表 2,氨基酸组成见表 3。
|
|
表 1 鱼溶浆粉的营养组成及肽分子量分布(干物质基础) Table 1 Proximate composition and peptide molecular weight distribution of stickwater meal(DM basis) |
|
|
表 2 饲料配方及营养组成(干物质基础) Table 2 Formulation and proximate chemical composition of experimental diets (dry matter basis) |
|
|
表 3 实验饲料氨基酸组成(干物质基础) Table 3 Amino acids composition of experimental diets (dry matter basis) |
养殖实验在山东省海洋资源与环境研究院东营实验基地循环水养殖系统内进行,选用同一批次的珍珠龙胆石斑鱼幼鱼,用对照组(DF组)和D0组饲料驯养2周,驯养期间逐渐降低DF组比例,增加D0组比例,正式实验开始前挑选出540尾初始体重(Initial body weight, IBW)为(23.72±0.09) g大小均匀、体质健壮的珍珠龙胆石斑鱼幼鱼,随机分为6组,每组3个重复,每个重复30尾鱼。养殖实验进行56 d,每天定时定量(初始投喂量为体重的2%左右,根据摄食情况调整投喂量)投喂2次(7:30和16:30),投喂30 min后排残饵,数颗粒并计算残饵量。驯养及实验期间控制水温16~18 ℃,溶氧>6 mg/L,pH为7.6~8.2,氨氮 < 0.05 mg/L,亚硝酸氮 < 0.05 mg/L,光照周期为自然光周期。
1.3 样品采集实验结束后,禁食24 h,每桶鱼计数并称末体重(Final body weight, FBW),计算存活率和增重率。每桶取15尾鱼,测量体长、体重,计算肥满度,随机取5尾用于全鱼体成分分析,剩余10尾尾静脉取血后分离肌肉,所有操作均在冰盘内完成。血液于4 ℃静置4 h后离心(4 000 r/min, 10 min)取上清液。另每桶随机取3尾鱼,取前肠0.8 cm左右并固定于Bouin’s液中,24 h后转移到70%酒精中,经脱水、透明、浸蜡、包埋后,进行常规石蜡连续切片,切片厚度为7.0 μm,然后经H.E.染色后,中性树胶封片并编号,在徕卡高清摄像系统(LEICA ICC50 HD)下观察并拍照。
1.4 实验方法与计算公式增重率(Weight gain rate, WGR, %)=[末体重(g)-初体重(g)]×100/初体重(g);
特定生长率(Specific growth rate, SGR, %/d)=[ln末体重(g)-ln初体重(g)]×100/养殖天数(d);
饲料系数(Feed conversion ratio, FCR)=摄食饲料总量(g)/[末体重(g)-初体重(g)];
蛋白质效率(Protein efficiency ratio, PER)=[末体重(g)-初体重(g)]/[摄食饲料总量(g)×饲料蛋白含量(%)];
肝体比(Hepatosomatic index, HSI, %)=肝脏质量(g)×100/末体重(g);
脏体比(Viscerosomatic index, VSI, %)=内脏质量(g)×100/末体重(g);
肥满度(Condition factor, CF, g/cm3)=体重(g)×100/体长(cm)3;
存活率(Survival rate, SR, %)=成活尾数×100/总尾数。
鱼溶浆粉肽含量及分子量分布由中国广州分析测试中心检测(凝胶色谱法),饲料及背肌氨基酸采用HITACHI L-8900氨基酸分析仪测定;血清总抗氧化能力(Total antioxidant capacity, T-AOC)、超氧化物歧化酶(Superoxide dismutase, SOD)、丙二醛(Malondialdehyde, MDA)、酸性磷酸酶(Acid phosphatase, ACP)活性均采用南京建成生物工程研究所试剂盒测定;血清总蛋白(Total protein, TP)、白蛋白(Albumin, ALB)、甘油三酯(Triglyceride, TG)、胆固醇(Cholesterol, CHOL)、高密度脂蛋白胆固醇(High-density lipoprotein cholesterol, HDL-C)、低密度脂蛋白胆固醇(Low-density lipoprotein cholesterol, LDL-C)含量、谷丙转氨酶(Alanine transaminase, ALT)、谷草转氨酶(Aspartate Aminotransferase, AST)、碱性磷酸酶(Alkaline phosphatase, AKP)活性均采用日立全自动生化分析仪(7020型, Hitachi)测定,试剂购自北京利德曼生化股份有限公司。
1.5 数据分析所有数据采用SPSS 18.0进行单因素方差分析(One-way ANOVA),用Duncan’s检验进行多重比较分析,P<0.05认为差异显著,P>0.05认为差异不显著。统计数据以平均值±标准误(Means±SE)的形式表示。
2 结果 2.1 生长性能、饲料利用、形体指标及存活率由表 4可见,高植物蛋白饲料中添加一定量的鱼溶浆粉,能显著改善鱼粉含量低引起的珍珠龙胆石斑鱼幼鱼生长下降。D0和D2.5组的增重率和特定生长率显著低于对照组(P<0.05),当鱼溶浆粉添加量为5%以上时,增重率和特定生长率与对照组差异不显著(P>0.05);各组间饲料系数和蛋白质效率差异不显著(P>0.05);D5、D7.5、D10组肝体比与DF组差异不显著(P>0.05),但显著低于D0组(P<0.05),D2.5组与各组差异均不显著(P>0.05),D0和D2.5组脏体比显著高于其它各组(P<0.05);D0组肥满度显著低于DF组(P<0.05),添加鱼溶浆粉的各组与DF组差异不显著(P>0.05);不同处理组珍珠龙胆石斑鱼幼鱼的存活率差异不显著(P>0.05)。
|
|
表 4 高植物蛋白饲料中添加鱼溶浆粉对珍珠龙胆石斑鱼幼鱼生长性能、饲料利用、形体指标及存活率的影响 Table 4 Effects of stickwater meal supplementation in high plant protein diets on growth performance, feed utilization, morphological parameters and survival rate of juvenile pearl gentian |
由表 5可见,不同处理组之间全鱼水分、粗脂肪、粗灰分差异均不显著(P>0.05),添加鱼溶浆粉的各组全鱼粗蛋白均显著高于DF组及D0组(P<0.05);不同处理组间背肌水分、粗蛋白、粗脂肪及粗灰分均无显著差异(P>0.05)。
|
|
表 5 高植物蛋白饲料中添加鱼溶浆粉对珍珠龙胆石斑鱼幼鱼营养成分的影响 Table 5 Effects of stickwater meal supplementation in high plant protein diets on proximate composition of juvenile pearl gentian grouper |
由表 6可见,高植物蛋白饲料中添加鱼溶浆粉对石斑鱼幼鱼背肌各种氨基酸含量影响均不显著(P>0.05),对必需氨基酸总和、非必需氨基酸总和及总氨基酸影响也均不显著(P>0.05)。
|
|
表 6 高植物蛋白饲料中添加鱼溶浆粉对珍珠龙胆石斑鱼幼鱼背肌氨基酸组成的影响 Table 6 Effects of stickwater meal supplementation in high plant protein diets on amino acids of dorsal muscle of juvenile pearl gentian grouper |
高植物蛋白饲料中添加鱼溶浆粉对珍珠龙胆石斑鱼幼鱼血清生化指标的影响见表 7。由表 7可见,各处理组血清TP差异显著(P<0.05),D0组显著低于对照组(P<0.05),D2.5和D5组与对照组差异不显著(P>0.05),但显著高于D0组(P<0.05),D7.5组显著高于其它各组(P<0.05)。各组间白蛋白差异不显著(P>0.05)。D7.5和D10组的TG含量显著高于其它各组(P<0.05)。DF组CHOL最高,显著高于其它各组(P<0.05),D7.5和D10组显著高于D0组。DF组的HDL-C和LDL-C均显著高于其它各组(P<0.05),D2.5和D5组LDL-C显著低于D7.5和D10组(P<0.05),显著高于D0组(P<0.05)。D0组的ALT和AST最高,均显著高于DF和D10组(P<0.05),D2.5、D5和D7.5组之间差异不显著(P>0.05)。
|
|
表 7 高植物蛋白饲料中添加鱼溶浆粉对珍珠龙胆石斑鱼幼鱼血清生化指标的影响 Table 7 Effects of stickwater meal supplementation in high plant protein diets on serum biochemical indices of juvenile pearl gentian grouper |
由表 8可见,D5、D7.5和D10组T-AOC显著高于对照组(P<0.05),D10组最高,D2.5与对照组差异不显著(P>0.05),D0组显著低于其它各组(P<0.05)。D0组T-SOD与D2.5组差异不显著(P>0.05),显著低于其它各组(P<0.05)。D0与D2.5组MDA含量最高,显著高于对照组及D10组(P<0.05),D10组与对照组差异不显著(P>0.05)。各处理组间ACP和ALP无显著差异(P>0.05)。
|
|
表 8 高植物蛋白饲料中添加鱼溶浆粉对珍珠龙胆石斑鱼幼鱼血清非特异性免疫指标的影响 Table 8 Effects of stickwater meal supplementation in high plant protein diets on nonspecific immunity of juvenile pearl gentian grouper |
由表 9和图 1可见,随着鱼溶浆粉添加量的增加,珍珠龙胆石斑鱼幼鱼肠道皱襞数目呈先升高后降低趋势,D0组和D2.5组显著低于DF组(P<0.05),D5、D7.5及D10组与对照组差异不显著(P>0.05),以D7.5组最高。D0组皱襞高度显著低于其它各组(P<0.05),添加鱼溶浆粉的各组与对照组差异均不显著(P>0.05)。D0组肌层厚度最低,但与其它各组差异不显著(P>0.05)。
|
|
表 9 高植物蛋白饲料中添加鱼溶浆粉对珍珠龙胆石斑鱼幼鱼肠道形态指标的影响 Table 9 Effects of stickwater meal supplementation in high plant protein diets on intestinal morphology of juvenile pearl gentian grouper |
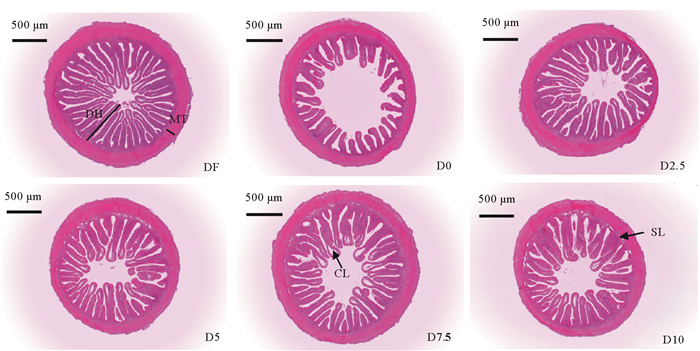
|
( DH:皱襞高度;MT:肌层厚度;CL:中央乳糜管;SL:黏膜下层。DH: Duplicature height; MT: Muscle thickness; CL: Central lacteal; SL: Submucous layer. ) 图 1 高植物蛋白饲料中添加鱼溶浆粉对珍珠龙胆石斑鱼幼鱼肠道组织结构的影响(×40) Fig. 1 Effects of stickwater meal replace white fishmeal in high plant protein diets on intestinal tissue structure of juvenile pearl gentian grouper(×40) |
海洋动物蛋白源含有的未知促生长因子可能是促进肉食性鱼类生长和提高饲料利用率的主要原因,植物蛋白源与海洋动物蛋白源相比,在小分子含氮化合物,如游离氨基酸、牛磺酸、游离核苷酸含量上存在明显差异[9]。本研究中,D0组的WGR、SGR及CF显著低于DF组,HSI和VSI显著高于DF组,随着鱼溶浆粉添加量的增加,WGR和SGR显著上升,添加5%以上时,与DF组无显著差异,HSI、VSI及CF也与对照组差异不显著,而本研究所用各实验饲料粗蛋白、粗脂肪、总能水平基本一致,据此推断,促进珍珠龙胆石斑鱼生长的因子可能是鱼溶浆粉中小的水溶性组分,这与大西洋鲑(Salmo salar)[10]、虹鳟(Oncorhynchus mykiss)[11]、大西洋鳕鱼(Gadus morhua)[12]的研究一致。对非洲鲶鱼(Clarias gariepinus)的研究表明,单独使用大豆产品替代鱼粉,替代量达到75%时(不额外添加晶体氨基酸),会影响实验鱼的生长性能和饲料利用效率[13],而用水解鱼蛋白替代鱼粉,非洲鲶鱼的WGR、饲料效率及CF均与鱼粉组无显著差异[14]。在含有15%和25%鱼粉的高植物蛋白饲料中,用水解鱼蛋白分别替代10%和15%的鱼粉蛋白,不会影响大菱鲆的生长性能[15-16]。在大西洋鳕鱼低鱼粉饲料中添加水解鱼蛋白后,其生长性能及饲料利用与鱼粉组无显著差异,超微过滤去除小分子化合物后,其饲料利用率降低[17],而在牙鮃(Paralichthys olivaceus)低鱼粉饲料中(30%鱼粉)添加3.7%的超微过滤的水解鱼蛋白,生长性能有所提升[18]。本研究5%以上的鱼溶浆粉即可替代30%的白鱼粉而对珍珠龙胆石斑鱼幼鱼的生长性能没有负面影响,表明海洋小分子化合物对于提高肉食性鱼类对植物蛋白源的利用率具有重要意义,肉食性鱼类对植物蛋白利用差的原因,一方面可能与含有的抗营养因子有关,另一方面可能缺乏一种或几种鱼类必需氨基酸或者矿物质,还可能是植物蛋白在消化过程中低分子肽的释放量和比例与鱼粉不同[3]。
本研究饲料粗蛋白水平基本一致,而D2.5、D5及D7.5组全鱼粗蛋白显著高于DF和D0组,D0组全鱼粗蛋白略低于DF组,但差异不显著,造成该结果的原因可能是鱼体对鱼溶浆粉中的小分子物质吸收利用能力较强,然而各鱼溶浆粉组的背肌营养组成与D0组及DF组差异均不显著,这与在大西洋鲑[10, 19]和大西洋鳕鱼[20]上的研究一致。鱼粉被认为是氨基酸配比最理想的饲料原料,植物蛋白源与鱼粉的差异会导致饲料氨基酸不平衡,本研究饲料氨基酸受原料影响较大,D0组Leu、Lys、Met、Thr含量均低于DF组,Asp、Cys及Glu高于DF组,D0组∑EAA低于DF组,添加鱼溶浆粉后,∑EAA有所升高,一定程度上弥补了植物蛋白引起的必需氨基酸缺乏,然而饲料氨基酸的差异并未引起肌肉氨基酸的差异,各实验组肌肉的四种主要呈味氨基酸Glu、Gly、Ala及Asp [21-22]含量均无显著差异。Aksnes等用全脂大豆、提取大豆、大豆浓缩蛋白和玉米蛋白的混合蛋白替代57.2%、73.9%和90.6%的饲料蛋白,虹鳟全鱼及背肌的氨基酸水平差异均不显著[17];Hevrøy等也发现,添加不同水平的水解鱼蛋白对大西洋鲑肌肉氨基酸组成无显著影响[23];而对大菱鲆(Scophthalmus maximus L.)的研究表明,在高植物蛋白饲料中用低分子水解鱼蛋白替代5%的鱼粉蛋白,肌肉氨基酸差异显著,背肌Phe、Lys、His及∑EAA/∑NEAA均显著高于其它各组[24],与本研究结果不一致,可见不同的养殖品种,饲料氨基酸对鱼体氨基酸的影响研究结果呈现较大差异。
鱼体血脂水平与新陈代谢和生理状况密切相关,CHOL和TG主要在肝脏中合成,其变化在一定程度上反映了肝脏对脂肪的代谢状况,含量升高表明动物内生脂肪转运活跃,是脂肪运输系统对高脂饲料的应答反应[25],CHOL还是动物合成胆汁酸、类固醇激素、肾上腺皮质激素、维生素D3等生理活性物质的前体[26-27]。在本研究中,随着鱼溶浆粉添加量的增加,血清CHOL含量显著增加,但均显著低于DF组,对牙鲆[28]、大西洋鳕鱼[20]及半滑舌鳎(Cynoglossus semi-laevis Günther)[29]的研究也表明,摄食高植物蛋白饲料后,实验鱼CHOL含量显著低于鱼粉组,XU等对大菱鲆的研究表明,用水解鱼蛋白替代鱼粉,血清TG、CHOL、HDL-C及LDL-C均降低[16],与本研究结果一致。LDL-C受体是一种跨膜糖蛋白,广泛分布于各种细胞和组织中,低密度脂蛋白将CHOL从肝脏运载至外周组织,供它们利用[30],血浆中的高密度脂蛋白可从外周组织将CHOL运送到肝内进行代谢,机体可以通过这种逆向转运把衰老细胞膜中的CHOL送到肝脏,再排到体外[31-32],在本研究中,与鱼粉对照组相比,高植物蛋白饲料组实验鱼的HDL-C及LDL-C均降低,随着鱼溶浆粉添加量的增加,LDL-C呈上升趋势,有研究报道,饲料蛋白源会影响血清CHOL水平,摄食大豆蛋白饲料的实验鱼血清CHOL和TG含量显著降低[31, 33],推测高植物蛋白饲料导致CHOL降低可能与其含有抗营养因子等非蛋白质组分有关,而适量的鱼溶浆粉能够帮助调节机体脂质代谢。
血清蛋白具有维持血液正常胶体渗透压和pH、运输多种代谢物、调节被运输物质的生理作用和解除其毒性、免疫以及营养等多种功能,而ALB具有结合和运输内源性与外源性物质,维持血液胶体渗透压,清除自由基,抑制血小板聚集和抗凝血等生理功能[34]。本研究中,D0组血清TP显著低于DF组,随着鱼溶浆粉添加量的增加,TP水平呈先升高后降低趋势,表明适量的鱼溶浆粉能增强鱼体蛋白质合成代谢[6]。本研究D0组血清ALT和AST活性均显著高于DF组,正常情况下,肝脏中ALT含量较高,血清中ALT含量很低,AST在心肌细胞中含量最高,肝脏次之,当肝脏组织病变,细胞坏死或通透性增强时,ALT和AST由肝脏释放到血清中[35-36],这表明,本研究珍珠龙胆石斑鱼摄食高植物蛋白饲料后,肝脏受到一定程度损伤,而随着鱼溶浆粉添加量的增加,血清中ALT和AST均有降低趋势,对肝脏起到保护作用,据此推测,高植物蛋白饲料中添加适量的鱼溶浆粉能帮助机体维持正常生理功能,提高机体免疫力。
在鱼蛋白水解产物中发现的多肽组分可能刺激水产动物重要的抗病因子,研究发现,不同水平和不同分子量的水解鱼蛋白均能影响欧洲鲈(Dicentrarchus labrax)稚鱼的生长和对环境中致病菌的抵抗能力[37];在饲料中添加水解鱼蛋白显著提高了欧洲鲈鱼血清T-SOD的活力、巨噬细胞活性、呼吸爆发、血清补体及免疫球蛋白等为标志的非特异性免疫力[38];在凡纳滨对虾(Litopenaeus vannamei)饲料中添加低分子的水解鱼蛋白,显著提高了肝胰腺中T-SOD的活力,降低了MDA含量[39],而MDA含量能反映机体脂质过氧化程度,间接反映细胞损伤程度;在牙鮃饲料中添加6%~16%的低分子水解鱼蛋白,牙鮃的T-AOC及T-SOD活性显著高于低鱼粉组[40]。与上述研究结果一致,在本研究中,D0组T-AOC和T-SOD活性最低,显著低于其它各组,随着鱼溶浆粉添加量的增加,添加量5%以上时,T-AOC显著高于鱼粉对照组,T-SOD与鱼粉组差异不显著,添加鱼溶浆粉10%时,MDA含量与鱼粉组差异不显著,可见,鱼溶浆粉对于提高低鱼粉饲料饲喂的珍珠龙胆石斑鱼幼鱼非特异性免疫性能、降低氧化产物具有重要作用,可能是因为鱼溶浆中的生物活性肽具有免疫刺激和抗菌作用,能提高机体免疫力[16]。
肠道的消化吸收能力与肠道组织结构密切相关,肠道皱襞高度和数目直接反映肠道的吸收面积[41],皱襞越高,数量越多,吸收面积越大,中央乳糜管的形态也与营养物质的吸收与运输密切相关[42]。本实验从肠道组织切片可以看出,DF组肠道皱襞均匀密集,用大豆浓缩蛋白替代50%的鱼粉后,D0组和D2.5组的肠道皱襞数目显著低于其他各组,皱襞短且排列稀疏,中央乳糜管变窄,影响肠道对营养物质的吸收,有研究表明,在大西洋鲑[43]、欧洲鲈[44]和斑马鱼(Barchydanio rerio)幼鱼[45]的饲料中添加一定量的大豆产品后,导致鱼后肠组织结构发生形态变化,并且肠黏膜发生炎症反应,这可能是由于小肠壁上皮细胞表面的特异性受体与大豆中的某些致敏原发生结合,导致肠黏膜出现炎症反应所致[46],这可能是造成鱼体生长下降的主要原因,而在高植物蛋白基础之上添加5%以上鱼溶浆粉后,皱襞高度和数目与DF组无显著差异,中央乳糜管变宽,对营养物质的吸收能力增强,可能是因为鱼溶浆粉中含有较多的牛磺酸(13.24 g/kg),而有研究表明,牛磺酸能增加肠道皱襞高度,降低隐窝深度,增加杯状细胞数量,对于修复肠道具有重要作用[47],本研究随着饲料中鱼溶浆粉添加量的增加,牛磺酸含量升高,因此推断,鱼溶浆粉能修复高植物蛋白给肠道结构带来的损伤。
4 结语本研究结果表明,鱼溶浆粉在珍珠龙胆石斑鱼饲料中具有多重应用效果,一方面可以作为营养物质替代鱼粉,另一方面可以调节机体脂质代谢,还可以作为免疫增强剂刺激鱼体非特异性免疫性能,改善肠道健康。因此,鱼溶浆粉应用于肉食性鱼类高植物蛋白饲料可以有效降低鱼粉添加量,有利于水产养殖业的可持续发展。
| [1] |
周歧存, 麦康森, 刘永坚, 等. 动植物蛋白源替代鱼粉研究进展[J]. 水产学报, 2005, 29(3): 404-410. Zhou Q C, Mai K S, Liu Y J, et al. Advances in animal and plant protein sources in place of fish meal[J]. Journal of Fisheries of China, 2005, 29(3): 404-410. (  0) 0) |
| [2] |
Francis G, Makkar H P S, Becker K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish[J]. Aquaculture, 2001, 199(3): 197-227.
(  0) 0) |
| [3] |
郑珂珂, 梁萌青, 姚宏波, 等. 在高植物蛋白饲料中添加水解鱼蛋白对牙鲆幼鱼的影响[J]. 水生生物学报, 2011, 35: 829-834. Zheng K K, Liang M Q, Yao H B, et al. Inclusion of size-fractionated fish protein hydrolysate in high plant protein diets for japanese flounder, Paralichthys olivaceus[J]. Acta Hydrobiologica Sinica, 2011, 35: 829-834. (  0) 0) |
| [4] |
Aksnes A, Mundheim H, Toppe J, et al. The effect of dietary hydroxyproline supplementation on salmon (Salmo salar L.) fed high plant protein diets[J]. Aquaculture, 2008, 275(1-4): 242-249. DOI:10.1016/j.aquaculture.2007.12.031
(  0) 0) |
| [5] |
Olsen R L, Toppe J. Fish silage hydrolysates: Not only a feed nutrient, but also a useful feed additive[J]. Trends in Food Science & Technology, 2017, 66: 93-97.
(  0) 0) |
| [6] |
吴代武, 叶元土, 蔡春芳, 等. 鱼溶浆粉替代鱼粉对草鱼生长及健康的影响[J]. 动物营养学报, 2015, 27: 2094-2105. WU D W, YE Y T, CAI C F, et al. Effects of fish meal replacement by stickwater meal on growth and health of grass carp (Ctenopharyngodon idellus)[J]. Chinese Journal of Animal Nutrition, 2015, 27: 2094-2105. (  0) 0) |
| [7] |
李学丽, 王际英, 宋志东, 等. 酶解豆粕替代鱼粉对珍珠龙胆石斑鱼幼鱼生长和主要代谢酶活力的影响[J]. 海洋渔业, 2017, 39(5): 529-538. LI X L, WANG J Y, SONG Z D, et al. Effects of fishmeal replacement by hydrolyzed soybean meal on growth, body composition, digestive and metabolic enzyme activities of juvenile Epinephelus fuscoguttatus♀×Epinephelus lanceolatus[J]. Marine Fisheries, 2017, 39(5): 529-538. (  0) 0) |
| [8] |
魏佳丽, 王际英, 宋志东, 等. 酶解磷虾粉替代鱼粉对珍珠龙胆石斑鱼幼鱼生长性能、体组成及血清生化的影响[J]. 渔业科学进展, 2016, 37: 100-110. WEI J L, WANG J Y, SONG Z D, et al. Efects of the partial substitute for fish meal by hydrolyzed krill meal on growth perofrmance, the body composition and the serum biochemical prameters of juvenile pearl gentian grouper[J]. Progress in Fishery Sciences, 2016, 37: 100-110. (  0) 0) |
| [9] |
Anders A. Feed ingredients: The impacts of nitrogen extractives in aqua feed ingredients[J]. International Aqua Feed, 2005, 8: 28-30.
(  0) 0) |
| [10] |
Kousoulaki K, Albrektsen S, Langmyhr E, et al. The water soluble fraction in fish meal (stickwater) stimulates growth in Atlantic salmon (Salmo salar L.) given high plant protein diets[J]. Aquaculture, 2009, 289(1-2): 74-83. DOI:10.1016/j.aquaculture.2008.12.034
(  0) 0) |
| [11] |
Aksnes A, Hope B, Jönsson E, et al. Size-fractionated fish hydrolysate as feed ingredient for rainbow trout (Oncorhynchus mykiss) fed high plant protein diets. Ⅰ: Growth, growth regulation and feed utilization[J]. Aquaculture, 2006, 261(1): 305-317.
(  0) 0) |
| [12] |
Aksnes A, Hope B, Høstmark Ø, et al. Inclusion of size fractionated fish hydrolysate in high plant protein diets for Atlantic cod, Gadus morhua[J]. Aquaculture, 2006, 261(3): 1102-1110. DOI:10.1016/j.aquaculture.2006.07.038
(  0) 0) |
| [13] |
Fagbenro B O A, Davies S J. Use of soybean flour (dehulled, solvent-extracted soybean) as a fish meal substitute in practical diets for African catfish, Clarias gariepinus (Burchell 1822)[J]. Journal of Applied Ichthyology, 2001, 17: 64-69. DOI:10.1046/j.1439-0426.2001.00252.x
(  0) 0) |
| [14] |
Swanepoel J C, Goosen N J. Evaluation of fish protein hydrolysates in juvenile African catfish (Clarias gariepinus) diets[J]. Aquaculture, 2018, 496: 262-269. DOI:10.1016/j.aquaculture.2018.06.084
(  0) 0) |
| [15] |
Wei Y, Liang M, Mu Y, et al. The effect of ultrafiltered fish protein hydrolysate level on growth performance, protein digestibility and mRNA expression of PepT1 in juvenile turbot (Scophthalmus maximus L.)[J]. Aquaculture Nutrition, 2016, 22(5): 1006-1017. DOI:10.1111/anu.12319
(  0) 0) |
| [16] |
Xu H, Mu Y, Zhang Y, et al. Graded levels of fish protein hydrolysate in high plant diets for turbot (Scophthalmus maximus): Effects on growth performance and lipid accumulation[J]. Aquaculture, 2016, 454: 140-147. DOI:10.1016/j.aquaculture.2015.12.006
(  0) 0) |
| [17] |
Aksnes A, Hope B, Albrektsen S. Size-fractionated fish hydrolysate as feed ingredient for rainbow trout (Oncorhynchus mykiss) fed high plant protein diets. Ⅱ: Flesh quality, absorption, retention and fillet levels of taurine and anserine[J]. Aquaculture, 2006, 261(1): 318-326. DOI:10.1016/j.aquaculture.2006.07.026
(  0) 0) |
| [18] |
Zheng K, Liang M, Yao H, et al. Effect of dietary fish protein hydrolysate on growth, feed utilization and IGF-I levels of Japanese flounder (Paralichthys olivaceus)[J]. Aquaculture Nutrition, 2012, 18(3): 297-303. DOI:10.1111/j.1365-2095.2011.00896.x
(  0) 0) |
| [19] |
Mundheim H, Aksnes A, Hope B. Growth, feed efficiency and digestibility in salmon (Salmo salar L.) fed different dietary proportions of vegetable protein sources in combination with two fish meal qualities[J]. Aquaculture, 2004, 237(1-4): 315-331. DOI:10.1016/j.aquaculture.2004.03.011
(  0) 0) |
| [20] |
Hansen A C, Rosenlund G, Karlsen Ø, et al. Total replacement of fish meal with plant proteins in diets for Atlantic cod (Gadus morhua L.) I — Effects on growth and protein retention[J]. Aquaculture, 2007, 272(1-4): 599-611. DOI:10.1016/j.aquaculture.2007.08.034
(  0) 0) |
| [21] |
王际英, 张德瑞, 马晶晶, 等. 珍珠龙胆石斑鱼肌肉营养成分分析与品质评价[J]. 海洋湖沼通报, 2015(4): 61-69. Wang J Y, Zhang D R, Ma J J, et al. Nutritional components analysis and nutritive value evaluation of Epinephelus fuscoguttatus♀×E. lanceolatus muscles[J]. Transactions of Oceanology and Limnology, 2015(4): 61-69. (  0) 0) |
| [22] |
陈星星, 柯爱英, 潘齐存, 等. 珍珠龙胆石斑鱼营养成分分析与品质评价[J]. 海洋湖沼通报, 2018(1): 90-95. Chen X X, Ke A Y, Pan Q C, et al. Nutritional components analysis and nutritive value evaluation of Epinephelus fuscoguttatus♀×E. lanceolatus muscles[J]. Transactions of Oceanology and Limnology, 2018(1): 90-95. (  0) 0) |
| [23] |
Hevroy E M, Espe M, Waagbo R, et al. Nutrient utilization in Atlantic salmon (Salmo salar L.) fed increased levels of fish protein hydrolysate during a period of fast growth[J]. Aquaculture Nutrition, 2005, 11(4): 301-313. DOI:10.1111/j.1365-2095.2005.00357.x
(  0) 0) |
| [24] |
牟玉超, 梁萌青, 郑珂珂, 等. 高植物蛋白饲料中不同水平低分子水解鱼蛋白对大菱鲆(Scophthalmus maximus L.)幼鱼生长及肝脏IGF-I受体表达的影响[J]. 渔业科学进展, 2016, 37: 49-57. Mu Y C, Liang M Q, Zheng K K, et al. Effects of small molecule weight fish protein hydrolysate in high plant protein diets on the expression of liver IGF-I receptor and the growth of juvenile turbot (Scophthalmus maximus L.)[J]. Progress in Fishery Sciences, 2016, 37: 49-57. (  0) 0) |
| [25] |
Deng J, Mai K, AI Q, et al. Interactive effects of dietary cholesterol and protein sources on growth performance and cholesterol metabolism of Japanese flounder (Paralichthys olivaceus)[J]. Aquaculture Nutrition, 2010, 16(4): 419-429.
(  0) 0) |
| [26] |
程汉良, 夏德全, 吴婷婷. 鱼类脂类代谢调控与脂肪肝[J]. 动物营养学报, 2006, 18: 294-298. Cheng H L, Xia D Q, Wu T T. Fatty liver and regulation of lipids metabolism in fish[J]. Chinese Journal of Animal Nutrition, 2006, 18: 294-298. (  0) 0) |
| [27] |
Hernández P V, Olvera-Novoa M A, Rouse D B. Effect of dietary cholesterol on growth and survival of juvenile redclaw crayfish Cherax quadricarinatus under laboratory conditions[J]. Aquaculture, 2004, 236(1-4): 405-411. DOI:10.1016/j.aquaculture.2003.12.005
(  0) 0) |
| [28] |
陈京华.微生物发酵、外源酶制剂和促摄食物质对牙鲆(Paralichthys olivaceus)利用豆粕蛋白的影响[D].青岛: 中国海洋大学, 2006. Chen J H. Effects of Fermentation, Exogenous Enzyme and Feeding Stimulants on Utilization of Soybean Meal Protein by Japanese Flounder (Paralichthys olivaceus)[D]. Qingdao: Ocean University of China, 2006. (  0) 0) |
| [29] |
代伟伟.半滑舌鳎赖氨酸需求及其蛋白源替代研究[D].青岛: 中国海洋大学, 2011. Dai W W. Lysine Requirement and Fish Meal Replacement in Diets of Tongue Sole, Cynoglossus semilaevis Günther[D]. Qingdao: Ocean University of China, 2011. (  0) 0) |
| [30] |
Yun B, Ai Q, Mai K, et al. Synergistic effects of dietary cholesterol and taurine on growth performance and cholesterol metabolism in juvenile turbot (Scophthalmus maximus L.) fed high plant protein diets[J]. Aquaculture, 2012, 324-325: 85-91. DOI:10.1016/j.aquaculture.2011.10.012
(  0) 0) |
| [31] |
Dias J, Alvarez M J, Arzel J, et al. Dietary protein source affects lipid metabolism in the European seabass (Dicentrarchus labrax)[J]. Comp Biochem Physiol A Mol Integr Physiol, 2005, 142(1): 19-31. DOI:10.1016/j.cbpb.2005.07.005
(  0) 0) |
| [32] |
Deng J, Mai K, Ai Q, et al. Interactive effects of dietary cholesterol and protein sources on growth performance and cholesterol metabolism of Japanese flounder (Paralichthys olivaceus)[J]. Aquaculture Nutrition, 2009, 16(4): 419-429. DOI:10.1111/j.1365-2095.2009.00681.x
(  0) 0) |
| [33] |
Romarheim O H, Skrede A, Gao Y, et al. Comparison of white flakes and toasted soybean meal partly replacing fish meal as protein source in extruded feed for rainbow trout (Oncorhynchus mykiss)[J]. Aquaculture, 2006, 256(1-4): 354-364. DOI:10.1016/j.aquaculture.2006.02.006
(  0) 0) |
| [34] |
Khan A, Shah N, Muhammad M, et al. Quantitative determination of lethal concentration Lc50 of atrazine on biochemical parameters; total protein and serum albumin of freshwater fish grass carp (Ctenopharyngodon idella)[J]. Polish Journal of Environmental Studies, 2016, 25(4): 1555-1561. DOI:10.15244/pjoes/61849
(  0) 0) |
| [35] |
Song Z, Li H, Wang J, et al. Effects of fishmeal replacement with soy protein hydrolysates on growth performance, blood biochemistry, gastrointestinal digestion and muscle composition of juvenile starry flounder (Platichthys stellatus)[J]. Aquaculture, 2014, 426-427: 96-104. DOI:10.1016/j.aquaculture.2014.01.002
(  0) 0) |
| [36] |
Kouba A, Velisek J, Stara A, et al. Supplementation with sodium selenite and selenium-enriched microalgae biomass show varying effects on blood enzymes activities, antioxidant response and accumulation in common barbel (Barbus barbus)[J]. BioMed Research International, 2014, 2014(1): 143-146.
(  0) 0) |
| [37] |
Kotzamanis Y P, Gisbert E, Gatesoupe F J, et al. Effects of different dietary levels of fish protein hydrolysates on growth, digestive enzymes, gut microbiota, and resistance to Vibrio anguillarum in European sea bass (Dicentrarchus labrax) larvae[J]. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 2007, 147(1): 205-214.
(  0) 0) |
| [38] |
Liang M, Wang J, Chang Q, et al. Effects of different levels of fish protein hydrolysate in the diet on the nonspecific immunity of Japanese sea bass, Lateolabrax japonicus(Cuvieret Valenciennes, 1828)[J]. Aquaculture Research, 2006, 37(1): 102-106. DOI:10.1111/j.1365-2109.2005.01392.x
(  0) 0) |
| [39] |
李晓丽, 王玲, 宋凯, 等. 低鱼粉饲料中添加低分子水解鱼蛋白对凡纳滨对虾非特异性免疫力和抗氧化能力的影响[J]. 渔业科学进展, 2017, 38: 85-91. Li X L, Wang L, Song K, et al. Effects of dietary low-molecular-weight fish hydrolysate (LWFH) on nonspecific immunity and antioxidant capacity of pacific white shrimp (Litopenaeus vannamei)[J]. Progress in Fishery Sciences, 2017, 38: 85-91. (  0) 0) |
| [40] |
许团辉, 高湘萍, 梁萌青, 等. 高植物蛋白饲料中以低分子水解蛋白替代鱼粉对牙鲆生长性能及非特异性免疫的影响[J]. 渔业科学进展, 2012, 33: 60-69. Xu T H, Gao X P, Liang M Q, et al. Effects of amall size-fractionated fish protein hydrolysate substitution of fish meal in high plant protein diets on the growth performance and non-specific immunity of japanese flounder, Paralichthys olivaceus[J]. Progress in Fishery Sciences, 2012, 33: 60-69. (  0) 0) |
| [41] |
Yan L, Zhou X Q. Dietary glutamine supplementation improves structure and function of intestine of juvenile Jian Carp (Cyprinus carpio var. Jian)[J]. Aquaculture, 2006, 256(1-4): 389-394. DOI:10.1016/j.aquaculture.2006.02.011
(  0) 0) |
| [42] |
李宝山, 王际英, 张利民, 等. 发酵豆粕替代藻粉对仿刺参生长及消化生理的影响[J]. 海洋科学前沿, 2018, 5(2): 89-97. Li B S, Wang J Y, Zhang L M, et al. Effects of replacing algae powder by fermented soybean meal on growth and digestive physiology of juvenile sea cucumber Apostichopus japonicus Selenka[J]. Advances in Marine Sciences, 2018, 5(2): 89-97. (  0) 0) |
| [43] |
Refstie S, Storebakken T, Baeverfjord G, et al. Long-term protein and lipid growth of Atlantic salmon (Salmo salar) fed diets with partial replacement of fish meal by soy protein products at medium or high lipid level[J]. Aquaculture, 2001, 193: 91-106. DOI:10.1016/S0044-8486(00)00473-7
(  0) 0) |
| [44] |
Kaushik S J, Covès D, Dutto G, et al. Almost total replacement of fish meal by plant protein sources in the diet of a marine teleost, the European seabass, Dicentrarchus labrax[J]. Aquaculture, 2004, 230(1-4): 391-404. DOI:10.1016/S0044-8486(03)00422-8
(  0) 0) |
| [45] |
Hedrera M I, Galdames J A, Jimenez-Reyes M F, et al. Soybean meal induces intestinal inflammation in zebrafish larvae[J]. PLoS One, 2013, 8(7): 1-10.
(  0) 0) |
| [46] |
吴莉芳, 瞿子惠, 周锴, 等. 豆粕替代鱼粉对黄金鲈生长及肠道组织的影响[J]. 西北农林科技大学学报(自然科学版), 2017, 45: 1-8. Wu L F, Qu Z H, Zhou K, et al. Effects of replacing fish meal with soybean meal on growth and intestinal tissue of Perca flavescens[J]. Journal of Northwest A&F University(Natural Science Edition), 2017, 45: 1-8. (  0) 0) |
| [47] |
田芊芊, 胡毅, 毛盼, 等. 低鱼粉饲料中添加牛磺酸对青鱼幼鱼生长、肠道修复及抗急性拥挤胁迫的影响[J]. 水产学报, 2016, 40(9): 1330-1339. Tian Q Q, Hu Y, Mao P, et al. Effects of dietary taurine supplementation on growth, intestine structure and resistance to acute crowding stress in juvenile black carp (Mylopharyngodon piceus) fed low fish meal diets[J]. Journal of Fisheries of China, 2016, 40(9): 1330-1339. (  0) 0) |
2. National Demonstration Center for Experimental Fisheries Science Education, Shanghai Ocean University, Shanghai 201306, China
 2021, Vol. 51
2021, Vol. 51


