2. 青岛海洋科学与技术试点国家实验室海洋渔业科学与食物产出过程功能实验室,山东 青岛 266237;
3. 中国海洋大学三亚海洋研究院热带海洋生物种质资源开发与种业工程实验室,海南 三亚 572024
贝类是中国重要的海水养殖种类。随着水体富营养化和气候变化逐渐加剧,赤潮对滤食性双壳贝类的影响日益受到关注[1]。有毒赤潮又称为有害藻华[2](Harmful Algal Blooming,HABs),可产生麻痹性贝毒(PST)、腹泻型贝毒(Diarrhetic Shellfish Toxins,DST)、神经性贝毒(Neurotoxic Shellfish Toxins,NST)、失忆性贝毒(Amnesic Shellfish Toxins, AST)和西加毒素(Ciguatoxin,CTX)[3]。其中,PST广泛分布于世界各地的海洋和淡水生态系统中[4],被认为是严重危害人类健康的藻源毒素之一[5]。PST的致毒机理是通过高度专一地阻遏电压门控钠离子通道(Voltage-gated Sodium Channels,Nav),抑制细胞内动作电位的转导[6],从而引起恶心呕吐、肌肉麻痹、呼吸困难甚至窒息等症状[5]。双壳贝类通过滤食产毒藻,在体内蓄积上述毒素,并通过食物链进行传递,使人类和动物中毒[7]。近年来有关双壳贝类PST积累代谢及其调控机理的研究日渐深入,为理解贝类适应摄食产毒藻的遗传机制以及开展养殖贝类毒素积累防控提供了理论参考。本文就目前双壳贝类中PST积累和转化的研究进展进行综述,并对今后的重点研究方向进行展望。
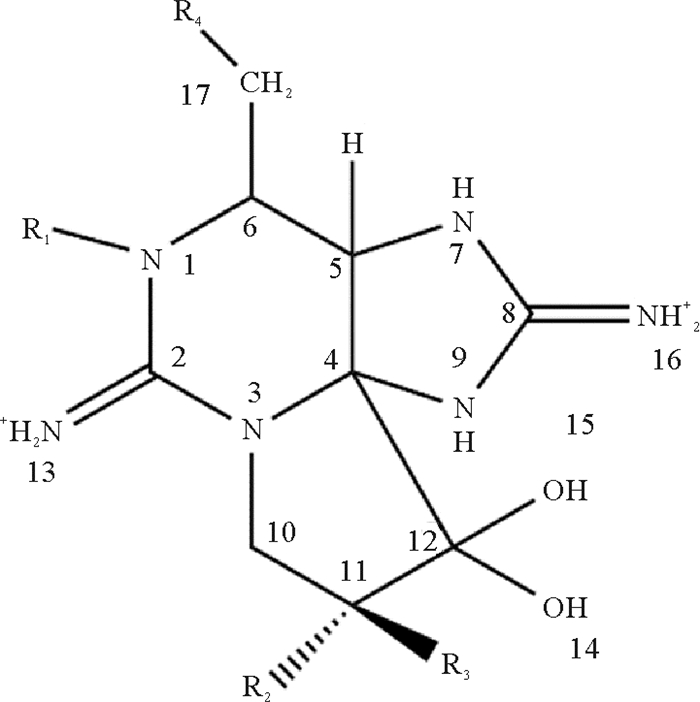
1 PST的种类与检测方法PST是一类四氢嘌呤衍生物(见图 1)的总称,甲藻和蓝藻是其主要来源[10]。在海洋环境中,PSTs主要由Alexandrium、Gymnodinium和Pyrodinium三个属的甲藻产生[11-13]。在淡水环境中,PSTs主要产自Anabaena、Aphanizomenon、Cylindrospermopsis、Lyngbya、Planktothrix和Rivularia等属的原核蓝藻[14-15]。地理位置、光照、水体盐度和环境温度等因素会对藻体中PST的合成产生影响[16-18],这导致了不同藻种以及不同藻株间PST种类和含量的显著差异[19-20]。
目前已鉴定到的PST衍生物有50余种[21],根据R4基团的不同主要分为四类(见表 1):①氨基甲酸酯类毒素(Carbamate toxins),包括石房蛤毒素(STX)、新石房蛤毒素(neoSTX)、膝沟藻毒素1-4(GTX1-4)、M2、M4等。②N-磺氨甲酰基类毒素(N-sulfocarbamoyl toxins),包括GTX5-6(B1-2)、C1-4、M1、M3等。③脱氨甲酰基类毒素(Decarbamoyl toxins)包括dcSTX、dcneoSTX、dcGTX1-4等。④脱氧脱氨甲酰基类毒素(Deoxydecarbamoyl toxins),包括doSTX、doGTX2、doGTX3等。其中,STX、GTX1和neoSTX毒力最高,C1、C2和GTX5的毒力最低。除此之外,一些比较罕见的R4侧链取代基,如羟基苯甲酸酯、硫酸苯甲酸酯和乙酸酯也陆续被鉴定和描述,但仅有部分的毒性被确定[22-25],新的STX类似物的不断发现使PST监测成为一项艰巨的任务。
|
|
表 1 麻痹性贝毒的代表性种类[21] Table 1 Representative analogs of paralytic shellfish toxin |
目前,PST检测方法主要有生物法、化学法和生物化学法三类。生物法又可分为小鼠生物检测法(MBA)、细胞检测法和免疫分析法。其中,MBA操作简便、应用广泛,是国际公认的PST检测方法[9, 26],但该方法存在灵敏度差、特异性低、假阳性高以及实验动物伦理问题等不足之处。细胞检测法基于PST能够特异性结合离子通道的原理,灵敏度远高于MBA[9],但由于组织培养周期长,无法确定PST的组成和含量等因素制约了其普遍推广。免疫分析法分为血液凝固、放射免疫法和酶联免疫吸附测定(ELISA),其中ELISA在贝类PST测定中最常用[9, 26]。该法基于抗原抗体结合反应,具有简便、快速、灵敏、重现性好等特点,适合PST的快速检测。不足之处是PST之间会产生交叉反应,且抗体一般针对主要成分建立,检测毒素种类受限,影响结果的准确性[26]。化学法主要利用高效液相色谱(HPLC)和液相色谱-质谱联用检测(LC-MS)[27-28]。HPLC技术在20世纪70年代被提出,利用PST在碱性条件下被氧化可产生荧光信号的原理,以二氧化硅为固定相,使用柱后衍生技术进行检测[9],具有灵敏度和准确性高等优点,然而该方法的样品前处理操作比较复杂。LC-MS法将色谱和质谱的优点相结合,比HPLC灵敏度更高,除了对已知PST进行定量定性分析外,还能发现未知毒素,已逐渐成为PST检测的主流方法[26]。生物化学检测法利用了新型的生物传感器技术,通过生物化学作用产生的高亲和力,将毒素的浓度转换为电信号进行检测[27-28]。常用的PST生物传感器主要有免疫生物传感器、毛细管电泳生物传感器以及核酸适配体生物传感器。该技术具有检测速度快、特异性好的优点,但在重复性和稳定性以及使用寿命方面还需进一步完善。
2 PST在双壳贝类中的积累 2.1 双壳贝类积累PST的种间差异作为PST的主要动物载体,双壳贝类在积累和清除PST方面有较大种间差异。贻贝可在短期内迅速积累和排出较高剂量的毒素[10],例如,淡水贻贝(Alathyria condola)暴露于有毒蓝藻(Anabaena circinalis)8 d其毒素水平最高可达570 μg STXeq 100 g-1[29]。翡翠贻贝(Perna viridis)摄食产毒亚历山大藻(Alexandrium fundyense)7 d后毒力水平达到最大值156 μg STXeq 100 g-1,而净化1 d后其肝胰腺中毒素含量就减少了50%[30]; 扇贝对PST敏感性低,积累速度快,保留时间长[10, 31]。暴露于微小亚历山大藻(Alexandrium minutum)48 h后的栉孔扇贝(Chlamys farreri)内脏团毒力值可达5 000 μg STXeq 100 g-1,经24 d的净化其毒力值仍高于食用安全标准[32]。加拿大芬迪湾的大西洋海扇贝(Placopecten magellanicus)全年PST浓度均高于食用安全标准,由于排出效率低,PST可在体内保留一年以上[33]; 牡蛎对PST敏感,因此积累毒素水平较低并能快速排出体外[34]。Mizuta等[35]对比了牡蛎、贻贝和扇贝的PST积累和清除能力,发现贻贝和扇贝的毒素积累量是牡蛎的3倍。Takata等[36]在日本濑户内海的双壳贝类中发现了同样的规律,长牡蛎(Crassostrea gigas)的最大毒力水平低于虾夷扇贝(Patinopecten yessoensis)和紫贻贝(Mytilus edulis),并且长牡蛎和紫贻贝分别经过14和30 d的净化已检测不到PST,而虾夷扇贝3个月后PST含量依旧很高。蛤蜊中,大部分种类毒素积累量较低,浓度也会迅速下降[37-39]。如短颈蛤(Tapes japonica)摄食有毒藻12 h后PST含量达到最大值,随后持续下降,到第7 d仅有0.6%的PST残留[37]。Lassus等[40]发现塔玛亚历山大藻(Alexandrium tamarense)暴露后菲律宾蛤仔(Ruditapes philippinarum)中PST积累速率和最大负荷(80 μg/100 g)显著低于紫贻贝(M. edulis)(1 100 μg/100 g)和欧洲扇贝(Pecten maximus)(2 700 μg/100 g)。但蛤类中也有PST慢速排出的物种,例如石房蛤(Saxidomus giganteus)和大西洋浪蛤(Spisula solidissima)中PST保留时间较长(约两年以上)[41]。
双壳贝类间积累PST的差异主要与以下三个方面的因素有关。首先,贝类积累毒素的能力主要取决于过滤速度、选择性摄取和吸收有毒细胞的能力[39],第二,双壳贝类在神经[42]、生理[43]和行为[44]反应中对毒素敏感性有差异。第三,PST在双壳贝类中的外排速率也是影响毒素累积的重要因素。
2.2 双壳贝类积累PST的组织器官差异已有研究证实PST在双壳贝类体内的分布具有组织特异性。双壳类的肝胰腺、肾、肠道积聚了80%~90%的毒素[45-48],而鳃、外套膜和闭壳肌的毒素水平相对较低[49]。Kwong等[27]研究发现翡翠贻贝(P. viridis)各组织PST含量依次为:肝胰腺>内脏>鳃>足和闭壳肌。Garcia等[50]对智利南部海域28个地点的贻贝、蛤蜊和竹蛏等双壳贝类进行了PST含量检测,也发现大部分物种的组织中PST浓度依次为肝胰腺>外套膜>闭壳肌。在其它物种如紫贻贝(M. edulis)、华贵栉孔扇贝(Chlamys nobilis)、海扇贝(P. magellanicus)、硬壳蛤(Mercenaria mercenaria)、砂海螂(Mya arenaria)、石房蛤(S. giganteus)、大西洋浪蛤(S. solidissima)、紫蛤(Hiatula rostrata)中均发现肝胰腺是毒素积累量最高的器官[10, 46, 51-53]。最近,Li等[54]对栉孔扇贝(C. farreri)的研究发现,肾的毒力水平比肝胰腺更高,二者分别是PST转化和吸收的主要器官,这一规律也在虾夷扇贝(P. yessoensis)中得到验证[55]。PST的选择性保留和转化能力不同是组织间毒性差异的主要原因[10]。
2.3 双壳贝类积累PST的分子机制大量研究发现双壳贝类在产毒甲藻繁盛时期仍具有良好的生存状态。与脊椎动物相比,双壳贝类具有积累和耐受高浓度PST的能力,主要归因于其Nav1具有耐毒氨基酸位点[54](见图 2)。河豚毒素(Tetrodotoxin,TTX)的致毒机理与PST相同,与双壳贝类Nav1中相似的耐毒位点也在河豚中发现(见图 2),显示物种间的趋同进化[54]。另一方面,抗氧化、解毒以及免疫相关基因在积累PST的贝类中诱导表达,可能与耐毒机制有关。例如,栉孔扇贝(C. farreri)和虾夷扇贝(P. yessoensis)中,超氧化物歧化酶(Superoxide dismutase,SOD)[56]、谷胱甘肽巯基转移酶(Glutathione S-transferase,GST)[57]、谷胱甘肽过氧化物酶(Glutathione peroxidases,GPX)[58]和热休克蛋白70(heat shock protein70,HSP70)[59-60]等基因家族发生扩张,这些扩张家族成员基因在产PST藻暴露后的扇贝中发生表达变化,且变化模式与产毒藻种类有关,也有组织器官特异性。注射STX后,贻贝(M. chilensis)中SOD、HSP70、过氧化氢酶(Catalase,CAT)、铁蛋白(Ferritin)以及参与免疫应答的多种模式识别受体基因的转录水平显著提高,这可能与贻贝应对STX积累的保护机制有关[61-62]。Mat等[63]对摄食微小亚历山大藻(A. minutum)的长牡蛎(C. gigas)进行了转录组分析,揭示了一系列与钠和钙交换相关途径,包括离子通道、神经肌肉信号交流、消化等都受到影响。此外,双壳贝类大量积累PST也与吸收和清除PST的机制有关[64]。如溶质转运蛋白(Solute Carrier,SLC)[65]和ABC转运蛋白(ATP-binding cassette transporter, ABC)[66]基因在扇贝中的扩张及其在PST积累过程中的多样化表达模式也提示,它们可能介导了PST的吸收和外排。进一步挖掘双壳贝类中与PST积累、代谢以及耐毒相关的基因有助于深入理解其摄食产PST藻的适应性进化机制。
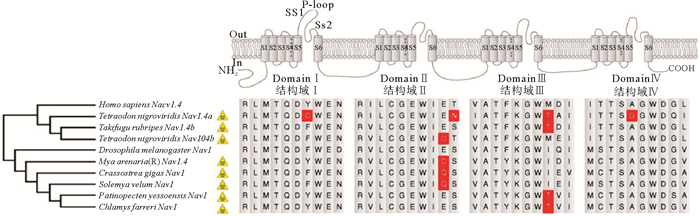
|
图 2 海洋动物钠通道Nav1具有PST或TTX抗性的氨基酸位点(红色突出)[54] Fig. 2 Amino acids (highlighted in red) potentially resistant to PST or TTX are found in sodium channel Nav1 of marine animals[54] |
贝类与摄入的产毒藻间PST组成有差异,这被认为是吸收后发生代谢转化的结果[67-68]。通常,贻贝和牡蛎中PST组成与摄入的产毒藻相一致,只是衍生物的相对比例发生改变[52, 69-71],例如Kwong等[27]在翡翠贻贝(P. viridis)中发现了与摄入毒藻相同的PST类似物,但贻贝组织中氨基甲酸酯毒素(GTXs)的比例有所增加。而在扇贝和蛤中产生了摄入藻中不存在的PST衍生物[72-74],提示其体内发生了PST的转化。Botelho等[75]研究了链状裸甲藻(Gymnodinium catenatum)暴露后贻贝(Mytilus galloprovincialis),鸟蛤(Cerastoderma edule)和竹蛏(Solen marginatus)中PST的积累差异发现,蛤和竹蛏对PST的转化效率比贻贝高。
在双壳贝类中普遍存在N-磺氨甲酰基类向氨基甲酸酯类毒素的转化。如暴露于产毒藻(A. fundyense)的翡翠贻贝(P. viridis)中发生了C类PST向GTX类的转化[27],在摄食塔玛亚历山大藻(A. tamarense)的牡蛎(C. gigas)和新西兰扇贝(Pecten novaezelandiae)中有同样发现[76-77]; 无齿蚌(Anodonta cygnea)摄入蓝藻(Aphanizomenon issatschenkoi)后,GTX5转化成为高毒力的STX[45],这种转化在摄食亚历山大藻(A. pacificum)的虾夷扇贝(P. yessoensis)肾中也有报道[55]。此外,蛤类中还会发生N -磺氨甲酰基类或氨基甲酸酯类转化成脱氨甲酰基类衍生物。如Sullivan等[72]发现自然环境下的短颈蛤(Protothaca staminea)主要含有脱氨甲酰基类衍生物; 暴露于链状裸甲藻(G. catenatum)后的象拔蚌(Panopea globosa)内脏中发生了由低毒力的C类转化为毒性较强的dcGTX2和dcSTX[78]。在中华蛤(Mactra chinensis)、日本蛤(Peronidia venulosa)、浪蛤(Spisula solida)中报道了C类转化成脱氨甲酰基类PST的关键酶,证实了这种转化是一种酶促反应[79-81]。
双壳贝类中一些新的PST衍生物不断被发现,这是毒素转化的重要证据。如Dell'aversano等[82]在加拿大沿岸的两种贻贝(M. edulis)和(Mytilus trossulus)中首次发现了新的PST类似物(M1~M5)。随后,Vale等[83]在葡萄牙海域链状裸甲藻(G. catenatum)污染的贻贝(Mytilus galloprovinciallis)、鸟蛤(C. edule)、文蛤(Ruditapes decussatus)中也发现了M类毒素。M类毒素被认为是贝类代谢PST的产物,因为它们在产毒藻中未被检测到。M1和M2是由C1/2和GTX2/3中的11-羟基硫酸基团转化为羟基形成的,M3和M4分别是M1和M2进一步羟基化的结果,化合物M5的结构尚未确定[84]。Li等[84]首次从中国黄海海域的扇贝和蛤中发现了新的代谢产物M6、M8和M10。最近,该团队证实了M类PST的化学转化途径M1→M3→M5,并首次确定了两条新的转化途径:M2→M4→M6和neoSTX→M10[85]。
3.2 双壳贝类对PST转化的组织特异性目前在双壳贝类多个组织器官中发现了PST的转化作用,比如肝胰腺、肾、鳃、肌肉、足。其中,肝胰腺和肾是PST积累的主要部位,也被认为是毒素代谢和转化最活跃的组织[86-87]。如暴露于塔玛亚历山大藻(A. tamarense)的新西兰扇贝(P. novaezelandiae)肝胰腺中发生了C2向GTX1的转化[76]; Fast等[88]对太平洋短颈蛤(P. staminea)的解剖组织进行了PST体外转化能力的评估,发现肝胰腺的生物转化能力最强; 浪蛤(S. solida)肝胰腺的体外匀浆可以迅速将GTXs、neoSTX以及STX转化成相应的脱氨甲酰基类衍生物[79]。但最近的研究发现,肾是双壳贝类PST转化的主要器官。在栉孔扇贝(C. farreri)和虾夷扇贝(P. yessoensis)的肾中发现了由低毒力的GTX类或C类PST转化成高毒力的neoSTX或STX[54-55]。除此之外,PST的转化也发生于PST含量较低的组织中。如体外实验表明海扇贝(P. magellanicus)的足和肌肉能够迅速将GTX1/4和neoSTX转化为STX[89]。Matias等[90]在葡萄牙阿威罗泻湖采集到的鸟蛤(C. edule)中发现了GTX6、dcSTX和GTX5在鳃中的生物转化。
3.3 双壳贝类对PST的转化机制双壳贝类体内PST的分布、积累和转化虽已被广泛报道,但转化机制尚不明确。PST之间的转化比较复杂[89, 91-93](见图 3),涉及的代谢途径包括脱硫(GTX1/4转化成neoSTX、GTX2/3转化成STX)、硫酸化(GTX1-4转化成C1-4)、水解(STX转化成dc-STX)、还原(GTX1/4转化成GTX2/3)和外异构化(GTX3/4异构化成GTX2/1,C2异构化成C1)。目前研究的转化机制主要分为三种,分别是化学转化、酶促转化和细菌转化。
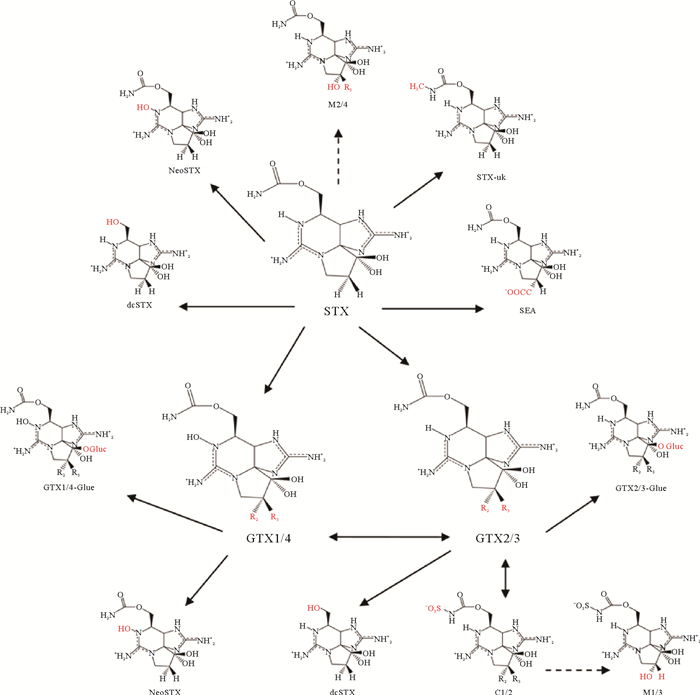
|
(红色突出显示与STX不同的基团,实线指示已证实的转化,虚线指示基于结构分析的假定转化。Moieties different from those in STX are highlighted in red. Unbroken line refers to confirmed toxin conversion. Broken line refers to putative transformation based on structural analysis. ) 图 3 麻痹性贝毒的转化途径[21] Fig. 3 Transformation pathway of the PST[21] |
Oshima[91]提出,双壳贝类中C11位有羟基硫酸基团的PST可以通过热力学平衡发生差向异构化,因此,毒藻中稳定的β型(GTX3/4和C2)毒素在双壳贝类中逐渐转化成更稳定的α型(GTX2/1和C1),直至两者比例达到1∶3[35, 94]。例如,塔玛亚历山大藻(A. tamarense)中占主导的是GTX4,而在摄食该藻后的蛤蜊、贻贝和牡蛎中则是GTX1占比最高[95]。此外贝类体内的天然还原剂(谷胱甘肽和半胱氨酸等)可通过还原N1位的羟基或C11位的硫酸基团使得氨基甲酸酯类衍生物发生相互转化[91]。其中,N1位羟基的还原使得GTX1/4转化成GTX2/3,这在日本蛤(Pseudocardium sachalinensis)、紫蛤(H. rostrata)、栉孔扇贝(C. farreri)中都有过报道[96-98]。在海扇贝(P. magellanicus)中,C11位的硫酸基团的消除可促使GTX1/4向neoSTX,GTX2/3向STX转化[89]。
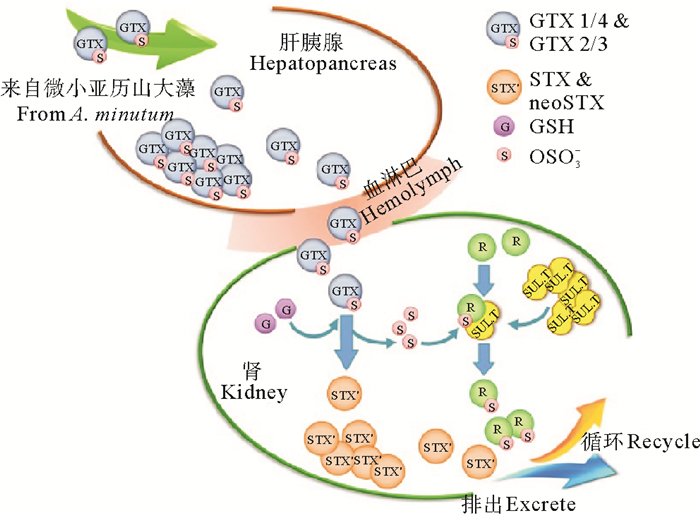
3.3.2 酶促转化酶促转化是双壳贝类转化PST的另一个重要途径[93]。目前已报道参与PST转化的酶有三种,分别是氨甲酰水解酶(Carbamoylase)、磺氨甲酰水解酶(Sulfocarbamoylase)和磺基转移酶(Sulfotransferase)。Lin等[81]首次从中华蛤(M. chinensis)的肝胰腺中分离纯化了氨甲酰水解酶Ⅰ,该酶的作用机理是催化氨基甲酸酯类或N-磺氨甲酰基类的R4基团水解生成脱氨甲酰基类毒素(见图 4)。Artigas等[79]通过体外实验在浪蛤(S. solida)中也检测到了氨甲酰水解酶活性。随后,Cho等[80]从日本蛤(P. venulosa)中首次分离纯化了磺氨甲酰水解酶Ⅰ,该酶只能通过水解磺氨甲酰基实现N-磺氨甲酰基类毒素向脱氨甲酰基类毒素的转化(见图 4)。此外,在扇贝的肾中,磺基转移酶可能参与PST的C11位硫酸基团转移,使GTX类PST转化为更高毒力的STX(见图 5)。
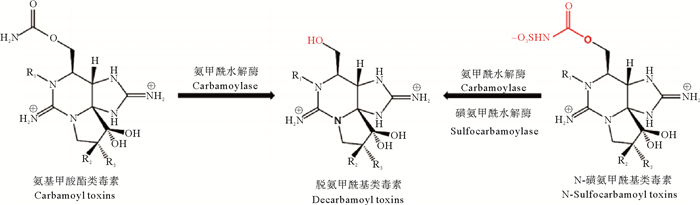
|
图 4 由氨甲酰水解酶和磺氨甲酰水解酶催化的PST转化[94] Fig. 4 Enzymatic transformations of PST catalyzed by carabamoylase I and sulfocarbamoylase I [94] |
|
图 5 栉孔扇贝肾中磺基转移酶介导的PST转化机制[54] Fig. 5 PST transformation putatively mediated by sulfotransferase in C. farreri kidney[54] |
除了自发的化学转化和酶促转化外,存在于双壳贝类消化系统中的细菌也可能参与了PST的转化和清除过程。Kotaki[99]首次从紫贻贝(M. edulis)体内分离出一种细菌,它能够还原性消除C11位上的硫酸基团和N1位的羟基,从而实现GTX类向高毒力的STX转化。最近Sakamoto等[100]发现细菌提取物将GTXs转化为STXs的活性是由于天然还原剂谷胱甘肽的存在。Donovan等[101]在污染的贻贝中分离并筛选出了7个菌株,能够使PST总毒性在3天内降低90%。Smith等[102]分别从贻贝,扇贝,牡蛎,蛤和蛏中分离出了C3、R69、M12、Q5、R78、K9、M7、M11和R65共9个菌株,并发现贝类中的细菌对PST的利用和转化能力不同。例如R65和M7对GTX2/3的利用率高,M12能够介导GTX1/4转化成GTX2/3,而所有这些菌中只有C3能够降解GTX5。目前关于细菌转化PST方面还存在争论,先前已经报道了酶促脱氨甲酰基化是细菌PST转化的潜在机制[102]。Sullivan等[72]把这种转化归因于贝类的酶,没有探索这些转化是细菌介导的可能性。贝类中由细菌介导的PST转化机制及其生物学意义有待更深入的研究。
4 总结与展望随着PST检测技术的提升和新的衍生物不断被发现,对双壳贝类积累转化PST的过程将有更进一步的认识。同时,多组学技术的广泛应用,也为系统鉴别PST积累转化关键基因提供了有力工具。进一步揭示PST积累转化过程中的重要分子途径和关键基因功能,将对深入理解双壳贝类摄食产PST藻的适应性进化机制,以及通过遗传改良降低双壳贝类PST积累有重要理论意义。
| [1] |
Trainer V L, Moore S K, Hallegraeff G, et al. Pelagic harmful algal blooms and climate change: Lessons from nature's experiments with extremes[J]. Harmful Algae, 2019, 91: 101591.
(  0) 0) |
| [2] |
Farabegoli F, Rodríguez L P, Vieites J M, et al. Phycotoxins in marine shellfish: Origin, occurrence and effects on humans[J]. Marine Drugs, 2018, 16(6): 188. DOI:10.3390/md16060188
(  0) 0) |
| [3] |
Grattan L M, Holobaugh S, Morris J G. Harmful algal blooms and public health[J]. Harmful Algae, 2016, 57: 2-8. DOI:10.1016/j.hal.2016.05.003
(  0) 0) |
| [4] |
Peacock M B, Gibble C M, Senn D B, et al. Blurred lines: multiple freshwater and marine algal toxins at the land-sea interface of San Francisco Bay, California[J]. Harmful Algae, 2018, 73: 138-147. DOI:10.1016/j.hal.2018.02.005
(  0) 0) |
| [5] |
Etheridge S M. Paralytic shellfish poisoning: Seafood safety and human health perspectives[J]. Toxicon, 2010, 56(2): 108-122. DOI:10.1016/j.toxicon.2009.12.013
(  0) 0) |
| [6] |
Llewellyn L E. Saxitoxin, a toxic marine natural product that targets a multitude of receptors[J]. Natural Product Reports, 2006, 23(2): 200-222. DOI:10.1039/b501296c
(  0) 0) |
| [7] |
James K J, Carey B, O'halloran J, et al. Shellfish toxicity: Human health implications of marine algal toxins[J]. Epidemiology and Infection, 2010, 138(7): 927-940. DOI:10.1017/S0950268810000853
(  0) 0) |
| [8] |
Gainey L G, Shumway S E. A compendium of the responses of bivalve molluscs to toxic dinoflagellates[J]. Journal of Shellfish Research, 1988, 7: 623-628.
(  0) 0) |
| [9] |
Tan K S, Ransangan J. Factors influencing the toxicity, detoxification and biotransformation of paralytic shellfish toxins[J]. Reviews of Environmental Contamination and Toxicology, 2015, 235: 1-25.
(  0) 0) |
| [10] |
Bricelj V M, Shumway S E. Paralytic shellfish toxins in bivalve molluscs: Occurrence, transfer kinetics, and biotransformation[J]. Reviews in Fisheries Science, 1998, 6(4): 315-383. DOI:10.1080/10641269891314294
(  0) 0) |
| [11] |
Lefebvre K A, Bill B D, Erickson A, et al. Characterization of intracellular and extracellular saxitoxin levels in both field and cultured Alexandrium spp. samples from Sequim Bay, Washington[J]. Marine Drugs, 2008, 6(2): 103-116. DOI:10.3390/md6020103
(  0) 0) |
| [12] |
Oshima Y, Blackburn S I, Hallegraeff G M. Comparative study on paralytic shellfish toxin profiles of the dinoflagellate Gymnodinium catenatum from three different countries[J]. Marine Biology, 1993, 116(3): 471-476. DOI:10.1007/BF00350064
(  0) 0) |
| [13] |
Usup G, Kulis D M, Anderson D M. Growth and toxin production of the toxic dinoflagellate Pyrodinium bahamense var. compressum in laboratory cultures[J]. Natural Toxins, 1994, 2(5): 254-262. DOI:10.1002/nt.2620020503
(  0) 0) |
| [14] |
Carmichael W W, Evans W R, Yin Q Q, et al. Evidence for paralytic shellfish poisons in the freshwater cyanobacterium Lyngbya wollei (Farlow ex Gomont) comb. nov[J]. Applied and Environmental Microbiology, 1997, 63(8): 3104-3110. DOI:10.1128/aem.63.8.3104-3110.1997
(  0) 0) |
| [15] |
Lagos N, Onodera H, Zagatto P A, et al. The first evidence of paralytic shellfish toxins in the fresh water cyanobacterium Cylindrospermopsis raciborskii, isolated from Brazil[J]. Toxicon, 1999, 37(10): 1359-1373. DOI:10.1016/S0041-0101(99)00080-X
(  0) 0) |
| [16] |
Mohamed Z A. Potentially harmful microalgae and algal blooms in the Red Sea: Current knowledge and Research needs[J]. Marine Environmental Research, 2018, 140: 234-242. DOI:10.1016/j.marenvres.2018.06.019
(  0) 0) |
| [17] |
Yao J, Jin W, Li D, et al. Geographical distribution and seasonal variation in paralytic shellfish toxins in the coastal water of the South China Sea[J]. Toxicon, 2019, 168: 67-75. DOI:10.1016/j.toxicon.2019.06.221
(  0) 0) |
| [18] |
Yoshida T, Sako Y, Uchida A. Geographic differences in paralytic shellfish poisoning toxin profiles among Japanese populations of Alexandrium tamarense and A. catenella (Dinophyceae)[J]. Phycological research, 2001, 49(1): 13-21. DOI:10.1111/j.1440-1835.2001.tb00228.x
(  0) 0) |
| [19] |
Franco J M, Fernández, P, Reguera B. The toxin profiles of natural populations and cultures of Alexandrium minutum Halim from Galician coastal waters[J]. Journal of Applied Phycology, 1994, 6(3): 275-279. DOI:10.1007/BF02181938
(  0) 0) |
| [20] |
Arzul G, Seguel M, Guzman L, et al. Comparison of allelopathic properties in three toxic Alexandrium species[J]. Journal of Experimental Marine Biology & Ecology, 1999, 232(2): 285-295.
(  0) 0) |
| [21] |
Wiese M, D'agostino P M, Mihali T K, et al. Neurotoxic alkaloids: Saxitoxin and its analogs[J]. Marine Drugs, 2010, 8(7): 2185-2211. DOI:10.3390/md8072185
(  0) 0) |
| [22] |
Minowa T, Cho Y, Oshima Y, et al. Identification of a novel saxitoxin analogue, 12beta-Deoxygonyautoxin 3, in the Cyanobacterium, Anabaena circinalis (TA04)[J]. Toxins, 2019, 11(9): 539. DOI:10.3390/toxins11090539
(  0) 0) |
| [23] |
Negri A, Stirling D, Quilliam M, et al. Three novel hydroxybenzoate saxitoxin analogues isolated from the dinoflagellate Gymnodinium catenatum[J]. Chemical Research in Toxicology, 2003, 16(8): 1029-1033. DOI:10.1021/tx034037j
(  0) 0) |
| [24] |
Onodera H, Satake M, Oshima Y, et al. New saxitoxin analogues from the freshwater filamentous cyanobacterium Lyngbya wollei[J]. Natural Toxins, 1997, 5(4): 146-151.
(  0) 0) |
| [25] |
Vale P. Complex profiles of hydrophobic paralytic shellfish poisoning compounds in Gymnodinium catenatum identified by liquid chromatography with fluorescence detection and mass spectrometry[J]. Journal of Chromatography A, 2008, 1195(1-2): 85-93. DOI:10.1016/j.chroma.2008.04.073
(  0) 0) |
| [26] |
黄爱君, 黄海燕, 刘建军. 麻痹性和腹泻性贝类毒素的检测方法研究进展[J]. 环境与健康杂志, 2010(1): 84-86. Huang A J, Huang H Y, Liu J J. Research progress on detection methods of paralytic and diarrhoeal shellfish toxins[J]. Journal of Environment and Health, 2010(1): 84-86. (  0) 0) |
| [27] |
张杭君, 张建英. 麻痹性贝毒素的毒理效应及检测技术[J]. 海洋环境科学, 2003(4): 76-80. Zhang H J, Zhang J Y. Toxicological effect and detection technology of paralytic shellfish toxin[J]. Marine Environmental Science, 2003(4): 76-80. DOI:10.3969/j.issn.1007-6336.2003.04.018 (  0) 0) |
| [28] |
汤云瑜, 黄冬梅, 蔡友琼. 麻痹性贝类毒素检测技术研究[J]. 农产品质量与安全, 2020, 108(6): 30-35. Tang Y Y, Huang D M, Cai Y Q. Detection of paralytic shellfish toxins[J]. Quality and Safety of Agro-Products, 2020, 108(6): 30-35. (  0) 0) |
| [29] |
Negri A P, Jones G J. Bioaccumulation of paralytic shellfish poisoning (PSP) toxins from the cyanobacterium Anabaena circinalis by the freshwater mussel Alathyria condola[J]. Toxicon, 1995, 33(5): 667-678. DOI:10.1016/0041-0101(94)00180-G
(  0) 0) |
| [30] |
Kwong R W, Wang W X, Lam P K, et al. The uptake, distribution and elimination of paralytic shellfish toxins in mussels and fish exposed to toxic dinoflagellates[J]. Aquatic Toxicology, 2006, 80(1): 82-91. DOI:10.1016/j.aquatox.2006.07.016
(  0) 0) |
| [31] |
Bing X F, Wu H Y, Wang Q, et al. Metabolic profile of paralytic shellfish toxin in scallop Chlamys farreri[J]. Journal of Fishery Sciences of China, 2017, 24: 623-632. DOI:10.3724/SP.J.1118.2017.16331
(  0) 0) |
| [32] |
朱明远, 邹迎麟, 吴荣军, 等. 栉孔扇贝体内麻痹性贝毒的累积与排出过程研究[J]. 海洋学报(中文版), 2003, 25(2): 75-83. Zhu M Y, Zou Y L, Wu R J, et al. Accumulation and depuration of paralytic shellfish poisons(PSP) in Chinese scallop Chlamys farreri[J]. Acta Oceanologica Sinica, 2003, 25(2): 75-83. DOI:10.3321/j.issn:0253-4193.2003.02.009 (  0) 0) |
| [33] |
Haya K, Martin J L, Robinson S, et al. Does uptake of Alexandrium fundyense cysts contribute to the levels of PSP toxin found in the sea scallop, Placopecten magellanicus?[J]. Harmful Algae, 2003, 2(1): 75-81. DOI:10.1016/S1568-9883(02)00068-9
(  0) 0) |
| [34] |
Xie W, Liu X, Yang X, et al. Accumulation and depuration of paralytic shellfish poisoning toxins in the oyster Ostrea rivularis Gould-Chitosan facilitates the toxin depuration[J]. Food Control, 2013, 30(2): 446-452. DOI:10.1016/j.foodcont.2012.07.035
(  0) 0) |
| [35] |
Mizuta M, Yamada K, Takata K, et al. Differences of accumulation and elimination of paralytic shellfish poisons among oyster, mussel and scallop[J]. Food Hygiene and Safety Science, 1991, 40(1): 19-22.
(  0) 0) |
| [36] |
Takata K, Seno M, Toukubo Y, et al. Differences in accumulation and elimination of paralytic shellfish toxins among oyster, scallop and mussel[J]. Nippon Suisan Gakkaishi, 2004, 70(4): 598-606. DOI:10.2331/suisan.70.598
(  0) 0) |
| [37] |
Samsur M, Yamaguchi Y, Sagara T, et al. Accumulation and depuration profiles of PSP toxins in the short-necked clam Tapes japonica fed with the toxic dinoflagellate Alexandrium catenella[J]. Toxicon, 2006, 48(3): 323-330. DOI:10.1016/j.toxicon.2006.06.002
(  0) 0) |
| [38] |
Asakawa M, Beppu R, Tsubota M, et al. Paralytic shellfish poison (PSP) profiles and toxification of short-necked clams fed with the toxic dinoflagellate Alexandrium tamarense[J]. Shokuhin Eiseigaku Zasshi, 2005, 46(6): 251-255. DOI:10.3358/shokueishi.46.251
(  0) 0) |
| [39] |
Li S C, Wang W X, Hsieh D. Feeding and absorption of the toxic dinoflagellate Alexandrium tamarense by two marine bivalves from the South China Sea[J]. Marine Biology, 2001, 139(4): 617-624. DOI:10.1007/s002270100613
(  0) 0) |
| [40] |
Lassus P, Fremy J M, Ledoux M, et al. Patterns of experimental contamination by Protogonyaulax tamarensis in some French commercial shellfish[J]. Toxicon Official Journal of the International Society on Toxinology, 1989, 27(12): 1313-1321. DOI:10.1016/0041-0101(89)90063-9
(  0) 0) |
| [41] |
Shumway S E, Cembella A D. The impact of toxic algae on scallop culture and fisheries[J]. Reviews in Fisheries Science, 1993, 1(2): 121-150. DOI:10.1080/10641269309388538
(  0) 0) |
| [42] |
Kvitek R G, Beitler M K. Relative insensitivity of butter clam neurons to saxitoxin: A pre-adaptation for sequestering paralytic shellfish poisoning toxins as a chemical defense[J]. Marine Ecology Progress Series, 1991, 69(1): 47-54.
(  0) 0) |
| [43] |
Navarro J M, Contreras A M. An integrative response by Mytilus chilensis to the toxic dinoflagellate Alexandrium catenella[J]. Marine Biology, 2010, 157(9): 1967-1974. DOI:10.1007/s00227-010-1465-x
(  0) 0) |
| [44] |
Basti L, Nagai K, Shimasaki Y, et al. Effects of the toxic dinoflagellate Heterocapsa circularisquama on the valve movement behaviour of the Manila clam Ruditapes philippinarum[J]. Aquaculture, 2009, 291(1-2): 41-47. DOI:10.1016/j.aquaculture.2009.02.029
(  0) 0) |
| [45] |
Pereira P, Dias E, Franca S, et al. Accumulation and depuration of cyanobacterial paralytic shellfish toxins by the freshwater mussel Anodonta cygnea[J]. Aquatic Toxicology, 2004, 68(4): 339-350. DOI:10.1016/j.aquatox.2004.04.001
(  0) 0) |
| [46] |
Cembella A D, Lewis N I, Shumway S E. Anatomical distribution and spatio-temporal variation in paralytic shellfish toxin composition in two bivalve species from the Gulf of Maine[J]. Journal of Shellfish Research, 1993, 12(2): 389-403.
(  0) 0) |
| [47] |
Song T, Liu L, Song X, et al. Depuration of paralytic shellfish toxins in Japanese scallop (Patinopecten yessoensis) in natural environment[J]. Acta Oceanologica Sinica, 2015, 34(12): 170-174. DOI:10.1007/s13131-015-0764-y
(  0) 0) |
| [48] |
田华, 张晓红, 高春蕾, 等. 麻痹性贝毒在栉孔扇贝体内短期的累积与排出过程[J]. 海洋环境科学, 2010(4): 521-524. Tian H, Zhang X H, Gao C L, et al. Short term accumulation and excretion of paralytic shellfish poison in Chlamys farreri[J]. Marine Environmental Science, 2010(4): 521-524. DOI:10.3969/j.issn.1007-6336.2010.04.016 (  0) 0) |
| [49] |
Cembella A D, Shumway S E, Larocque R. Sequestering and putative biotransformation of paralytic shellfish toxins by the sea scallop Placopecten magellanicus: Seasonal and spatial scales in natural populations[J]. Journal of Experimental Marine Biology and Ecology, 1994, 180(1): 1-22. DOI:10.1016/0022-0981(94)90075-2
(  0) 0) |
| [50] |
Garcia C, Perez F, Contreras C, et al. Saxitoxins and okadaic acid group: Accumulation and distribution in invertebrate marine vectors from Southern Chile[J]. Food Additives & Contaminants Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 2015, 32(6): 984-1002.
(  0) 0) |
| [51] |
Choi M C, Hsieh D, Lam P, et al. Field depuration and biotransformation of paralytic shellfish toxins in scallop Chlamys nobilis and green-lipped mussel Perna viridis[J]. Marine Biology, 2003, 143(5): 927-934. DOI:10.1007/s00227-003-1148-y
(  0) 0) |
| [52] |
Bricelj V M, Lee J H, Cembella A D, et al. Uptake kinetics of paralytic shellfish toxins from the dinoflagellate Alexandrium fundyense in the mussel Mytilus edulis[J]. Marine Ecology Progress Series, 1990, 63(2): 177-188.
(  0) 0) |
| [53] |
Bricelj V, Cembella A, Laby D M, et al. Comparative physiological and behavioral responses to PSP toxins in two bivalve molluscs, the softshell clam, Mya arenaria, and surfclam, Spisula solidissima[M]// Harmful & Toxic Algal Blooms. London: Informa Healthcare, 1996.
(  0) 0) |
| [54] |
Li Y, Sun X, Hu X, et al. Scallop genome reveals molecular adaptations to semi-sessile life and neurotoxins[J]. Nature Communications, 2017, 8(1): 1721. DOI:10.1038/s41467-017-01927-0
(  0) 0) |
| [55] |
Liu Y, Kong F Z, Xun X G, et al. Biokinetics and biotransformation of paralytic shellfish toxins in different tissues of Yesso scallops, Patinopecten yessoensis[J]. Chemosphere, 2020, 261: 128063. DOI:10.1016/j.chemosphere.2020.128063
(  0) 0) |
| [56] |
Lian S, Zhao L, Xun X, et al. Genome-wide identification and characterization of SODs in Zhikong scallop reveals gene expansion and regulation divergence after toxic dinoflagellate exposure[J]. Marine Drugs, 2019, 17(12): 700. DOI:10.3390/md17120700
(  0) 0) |
| [57] |
Lou J, Cheng J, Xun X, et al. Glutathione S-transferase genes in scallops and their diverse expression patterns after exposure to PST-producing dinoflagellates[J]. Marine Life Science & Technology, 2020, 2(3): 252-261.
(  0) 0) |
| [58] |
Hlaing S, Lou J, Cheng J, et al. Tissue-biased and species-specific regulation of Glutathione Peroxidase (GPx) genes in scallops exposed to toxic dinoflagellates[J]. Toxins, 2020, 13(1): 21. DOI:10.3390/toxins13010021
(  0) 0) |
| [59] |
Cheng J, Xun X, Kong Y, et al. Hsp70 gene expansions in the scallop Patinopecten yessoensis and their expression regulation after exposure to the toxic dinoflagellate Alexandrium catenella[J]. Fish & Shellfish Immunology, 2016, 58: 266-273.
(  0) 0) |
| [60] |
Hu B, Li M, Yu X, et al. Diverse expression regulation of Hsp70 genes in scallops after exposure to toxic Alexandrium dinoflagellates[J]. Chemosphere, 2019, 234: 62-69. DOI:10.1016/j.chemosphere.2019.06.034
(  0) 0) |
| [61] |
Núez-Acua G, Aballay A E, Hégaret H, et al. Transcriptional responses of Mytilus chilensis exposed in vivo to saxitoxin (STX)[J]. Journal of Molluscan Studies, 2013, 79(4): 323-331. DOI:10.1093/mollus/eyt030
(  0) 0) |
| [62] |
Detree C, Nunez-Acuna G, Roberts S, et al. Uncovering the complex transcriptome response of Mytilus chilensis against saxitoxin: Implications of harmful algal blooms on mussel populations[J]. Plos One, 2016, 11(10): e0165231. DOI:10.1371/journal.pone.0165231
(  0) 0) |
| [63] |
Mat A M, Klopp C, Payton L, et al. Oyster transcriptome response to Alexandrium exposure is related to saxitoxin load and characterized by disrupted digestion, energy balance, and calcium and sodium signaling[J]. Aquatic Toxicology, 2018, 199: 127-137. DOI:10.1016/j.aquatox.2018.03.030
(  0) 0) |
| [64] |
Botelho M J, Vale C, Ferreira J G. Profiles of paralytic shellfish toxins in bivalves of low and elevated toxicities following exposure to Gymnodinium catenatum blooms in Portuguese estuarine and coastal waters[J]. Chemosphere, 2015, 138: 1028-1036. DOI:10.1016/j.chemosphere.2014.12.072
(  0) 0) |
| [65] |
Xun X, Cheng J, Wang J, et al. Solute carriers in scallop genome: Gene expansion and expression regulation after exposure to toxic dinoflagellate[J]. Chemosphere, 2020, 241: 124968. DOI:10.1016/j.chemosphere.2019.124968
(  0) 0) |
| [66] |
Wang H, Liu S, Xun X, et al. Toxin- and species-dependent regulation of ATP-binding cassette (ABC) transporters in scallops after exposure to paralytic shellfish toxin-producing dinoflagellates[J]. Aquatic Toxicology, 2021, 230: 105697. DOI:10.1016/j.aquatox.2020.105697
(  0) 0) |
| [67] |
Blanco J, Ma I R, Franco J. Kinetics of accumulation and transformation of paralytic shellfish toxins in the blue mussel Mytilus galloprovincialis[J]. Toxicon Official Journal of the International Society on Toxinology, 2003, 42(7): 777-784. DOI:10.1016/j.toxicon.2003.10.007
(  0) 0) |
| [68] |
Yu K N, Kwong R, Wang W X, et al. Biokinetics of paralytic shellfish toxins in the green-lipped mussel, Perna viridis[J]. Marine Pollution Bulletin, 2007, 54(7): 1068-1071. DOI:10.1016/j.marpolbul.2007.02.007
(  0) 0) |
| [69] |
Onoue Y, Noguchi T, Maruyama J, et al. Comparison of PSP compositions between toxic oysters and Protogonyaulax catenella from Senzaki Bay, Yamaguchi Prefecture[J]. Nippon Suisan Gakkaishi, 1981, 47(10): 1347-1350. DOI:10.2331/suisan.47.1347
(  0) 0) |
| [70] |
Oshima Y, Hasegawa M, Yasumoto T, et al. Dinoflagellate Gymnodinium catenatum as the source of paralytic shellfish toxins in Tasmanian shellfish[J]. Toxicon Official Journal of the International Society on Toxinology, 1987, 25(10): 1105-1111. DOI:10.1016/0041-0101(87)90267-4
(  0) 0) |
| [71] |
Lassus P, Amzil Z, Baron R, et al. Modelling the accumulation of PSP toxins in Thau Lagoon oysters(Crassostrea gigas) from trials using mixed cultures of Alexandrium catenella and Thalassiosira weissflogii[J]. Aquatic Living Resources, 2007, 20(1): 59-67. DOI:10.1051/alr:2007016
(  0) 0) |
| [72] |
Sullivan J J, Iwaoka W T, Liston J. Enzymatic transformation of PSP toxins in the littleneck clam (Protothaca staminea)[J]. Biochemical and Biophysical Research Communications, 1983, 114(2): 465-472. DOI:10.1016/0006-291X(83)90803-3
(  0) 0) |
| [73] |
Murakami R, Yamamoto K, Noguchi T. Toxicity and paralytic shellfish poison composition of three species of bivalves collected in Ibaraki Prefecture, Japan[J]. Journal of the Food Hygienic Society of Japan, 2009, 40(1): 46-54.
(  0) 0) |
| [74] |
Botelho M J, Vale C, Grilo R V, et al. Uptake and release of paralytic shellfish toxins by the clam Ruditapes decussatus exposed to Gymnodinium catenatum and subsequent depuration[J]. Marine Environmental Research, 2012, 77: 23-29. DOI:10.1016/j.marenvres.2012.01.002
(  0) 0) |
| [75] |
Botelho M J, Marques F, Freitas R, et al. Paralytic shellfish toxin profiles in mussel, cockle and razor shell under post-bloom natural conditions: Evidence of higher biotransformation in razor shells and cockles[J]. Marine Environmental Research, 2020, 154: 104839. DOI:10.1016/j.marenvres.2019.104839
(  0) 0) |
| [76] |
Contreras A M, Marsden I D, Munro M. Physiological effects and biotransformation of PSP Toxins in the New Zealand scallop, Pecten novaezelandiae[J]. Journal of Shellfish Research, 2012, 31(4): 1151-1159. DOI:10.2983/035.031.0426
(  0) 0) |
| [77] |
Asakawa M, Beppu R, Ito K, et al. Accumulation of paralytic shellfish poison (PSP) and biotransformation of its components in oysters, Crassostrea gigas, fed with the toxic dinoflagellate Alexandrium tamarense[J]. Journal of the Food Hygienic Society of Japan, 2006, 47(1): 28-32. DOI:10.3358/shokueishi.47.28
(  0) 0) |
| [78] |
Medina-Elizalde J, García-Mendoza E, Turner A D, et al. Transformation and depuration of paralytic shellfish toxins in the geoduck clam Panopea globosa from the northern gulf of California[J]. Frontiers in Marine Science, 2018, 5: 335. DOI:10.3389/fmars.2018.00335
(  0) 0) |
| [79] |
Artigas M L, Vale P J, Gomes S S, et al. Profiles of paralytic shellfish poisoning toxins in shellfish from Portugal explained by carbamoylase activity[J]. Journal of Chromatography A, 2007, 1160(1-2): 99-105. DOI:10.1016/j.chroma.2007.04.008
(  0) 0) |
| [80] |
Cho Y, Ogawa N, Takahashi M, et al. Purification and characterization of paralytic shellfish toxin-transforming enzyme, sulfocarbamoylase I, from the Japanese bivalve Peronidia venulosa[J]. Biochimica et Biophysica Acta, 2008, 1784(9): 1277-1285. DOI:10.1016/j.bbapap.2008.05.008
(  0) 0) |
| [81] |
Lin H P, Cho Y, Yashiro H, et al. Purification and characterization of paralytic shellfish toxin transforming enzyme from Mactra chinensis[J]. Toxicon, 2004, 44(6): 657-668. DOI:10.1016/j.toxicon.2004.07.024
(  0) 0) |
| [82] |
Dell'aversano C, Walter J A, Burton I W, et al. Isolation and structure elucidation of new and unusual saxitoxin analogues from mussels[J]. Journal of Natural Products, 2008, 71(9): 1518-1523. DOI:10.1021/np800066r
(  0) 0) |
| [83] |
Vale P. Metabolites of saxitoxin analogues in bivalves contaminated by Gymnodinium catenatum[J]. Toxicon, 2010, 55(1): 162-165. DOI:10.1016/j.toxicon.2009.07.010
(  0) 0) |
| [84] |
Li A, Ma J, Cao J, et al. Analysis of paralytic shellfish toxins and their metabolites in shellfish from the North Yellow Sea of China[J]. Food Additives & Contaminants: Part A, 2012, 29(9): 1455-1464.
(  0) 0) |
| [85] |
Che Y, Ding L, Qiu J, et al. Conversion and stability of new metabolites of paralytic shellfish toxins under different temperature and pH conditions[J]. Journal of Agricultural and Food Chemistry, 2020, 68(5): 1427-1435. DOI:10.1021/acs.jafc.9b07063
(  0) 0) |
| [86] |
Lassus P, Bardouil M, Ledoux M, et al. Role of the kidneys in bioaccumulation of paralytic toxins by scallop (Pecten maximus) tissues[J]. Journal of Natural Toxins, 1996, 5(1): 107-115.
(  0) 0) |
| [87] |
Lu Y H, Hwang D F. Effects of toxic dinoflagellates and toxin biotransformation in bivalves[J]. Journal of Natural Toxins, 2002, 11(4): 315-322.
(  0) 0) |
| [88] |
Fast M D, Cembella A D, Ross N W. In vitro transformation of paralytic shellfish toxins in the clams Mya arenaria and Protothaca staminea[J]. Harmful Algae, 2006, 5(1): 79-90. DOI:10.1016/j.hal.2005.05.005
(  0) 0) |
| [89] |
Shimizu Y, Yoshioka M. Transformation of paralytic shellfish toxins as demonstrated in scallop homogenates[J]. Science, 1981, 212(4494): 547-549. DOI:10.1126/science.7209548
(  0) 0) |
| [90] |
Costa S T, Vale C, Raimundo J, et al. Changes of paralytic shellfish toxins in gills and digestive glands of the cockle Cerastoderma edule under post-bloom natural conditions[J]. Chemosphere, 2016, 149: 351-357. DOI:10.1016/j.chemosphere.2016.01.105
(  0) 0) |
| [91] |
Oshima Y. Chemical and Enzymatic Transformation of Paralytic Shellfish Toxins in Marine Organisms[M]. Paris: Harmful Marine Algal Blooms, 1995: 475-480.
(  0) 0) |
| [92] |
Ding L, Qiu J B, Li A F. Proposed biotransformation pathways for new metabolites of paralytic shellfish toxins based on field and experimental mussel samples[J]. Journal of Agricultural and Food Chemistry, 2017, 65(27): 5494-5502. DOI:10.1021/acs.jafc.7b02101
(  0) 0) |
| [93] |
Raposo M, Gomes M, Botelho M J, et al. Paralytic shellfish toxins (PST)-transforming enzymes: A review[J]. Toxins, 2020, 12(5): 344. DOI:10.3390/toxins12050344
(  0) 0) |
| [94] |
Choi M C, Yu P K, Hsieh D P, et al. Trophic transfer of paralytic shellfish toxins from clams (Ruditapes philippinarum) to gastropods (Nassarius festivus)[J]. Chemosphere, 2006, 64(10): 1642-1649. DOI:10.1016/j.chemosphere.2006.01.036
(  0) 0) |
| [95] |
Asakawa M, Miyazawa K, Takayama H, et al. Dinoflagellate Alexandrium tamarense as the source of paralytic shellfish poison (PSP) contained in bivalves from Hiroshima Bay, Hiroshima Prefecture, Japan[J]. Toxicon, 1995, 33(5): 691-697. DOI:10.1016/0041-0101(94)00177-A
(  0) 0) |
| [96] |
Murakami R, Yamamoto K, Noguchi T. Difference in PSP composition among various parts of surf clam[J]. Food Hygiene & Safety Science, 1999, 40(1): 55-61.
(  0) 0) |
| [97] |
Chen C Y, Chou H N. Accumulation and depuration of paralytic shellfish poisoning toxins by purple clam Hiatula rostrata Lighttoot[J]. Toxicon, 2001, 39(7): 1029-1034. DOI:10.1016/S0041-0101(00)00242-7
(  0) 0) |
| [98] |
Tian H, Gao C, Wang Z, et al. Comparative study on in vitro transformation of paralytic shellfish poisoning (PSP) toxins in different shellfish tissues[J]. Acta Oceanologica Sinica, 2010, 29(1): 120-126. DOI:10.1007/s13131-010-0015-1
(  0) 0) |
| [99] |
Kotaki Y. Screening of bacteria which convert gonyautoxin 2, 3 to Saxitoxin[J]. Nippon Suisan Gakkaishi, 1989, 55(7): 1293. DOI:10.2331/suisan.55.1293
(  0) 0) |
| [100] |
Sakamoto S, Sato S, Ogata T, et al. Formation of intermediate conjugates in the reductive transformation of gonyautoxins to saxitoxins by thiol compounds[J]. Fisheries Science, 2010, 66(1): 136-141.
(  0) 0) |
| [101] |
Donovan C J, Ku J C, Quilliam M A, et al. Bacterial degradation of paralytic shellfish toxins[J]. Toxicon, 2008, 52(1): 91-100. DOI:10.1016/j.toxicon.2008.05.005
(  0) 0) |
| [102] |
Smith E A, Grant F, Ferguson C M, et al. Biotransformations of paralytic shellfish toxins by bacteria isolated from bivalve molluscs[J]. Applied and Environmental Microbiology, 2001, 67(5): 2345-2353. DOI:10.1128/AEM.67.5.2345-2353.2001
(  0) 0) |
2. Laboratory for Marine Fisheries Science and Food Production Process, Pilot National Laboratory for Marine Science and Technology(Qingdao), Qingdao 266237, China;
3. Laboratory of Tropical Marine Germplasm Resources and Breeding Engineering, Sanya Oceanographic Institution, Ocean University of China, Sanya 572024, China
 2021, Vol. 51
2021, Vol. 51