2. 中国海洋大学海洋环境与生态教育部重点实验室,山东 青岛 266100
由于塑料廉价、耐用、强度高、耐腐蚀等特点,塑料产业在全球范围内迅速发展,各种材质的塑料制品广泛应用于产品包装、建筑、汽车、农业、医疗等领域[1]。据统计,全球每年塑料排放量约为2.4~3.4亿t,预计到2030年全球塑料年排放量可达5.3亿t。然而目前塑料的回收利用率不到10%,大量的塑料垃圾在环境中残留且难以降解,导致全球塑料污染问题日趋严峻[2]。基于细小塑料碎片或颗粒在环境中普遍存在,且潜在威胁生态系统和人体健康,科学家将最大维度小于5 mm的塑料颗粒或碎片统称为微塑料(Microplastics, MPs)[3]。微塑料在陆地、河流、海洋、极地甚至大气中广泛存在,能够通过多种途径进入生物体内,并可沿食物链传递,在生物组织和器官中蓄积和转移,产生发育毒性、行为毒性、生殖毒性、致死效应等不利影响[4-7],还可能诱导生物体产生炎症损伤、神经退行性疾病、免疫障碍等,潜在威胁人类健康[8-9]。
塑料是由高分子聚合物(合成树脂)及各种添加剂制成。塑料制品种类繁多,用途广泛,不同用途的塑料制品中添加剂的种类和添加量也有差别,如聚碳酸酯(Polycarbonate,PC)常添加双酚A(Bisphenol A,BPA)作为稳定剂[10];聚氯乙烯(Polyvinyl Chloride,PVC)常添加邻苯二甲酸酯类增塑剂(Phthalic Acid Esters,PAEs),添加量可高达60%[11];低密度聚乙烯(Low Density Polyethylene,LDPE)常添加酰胺类物质作为爽滑剂[12]。这些塑料添加剂伴随塑料的降解向外释放,特别是在高温和紫外线辐射导致塑料老化的变性过程中,大量的添加剂快速释放到环境中,危害生态环境和人体健康[13]。当前,微塑料问题是环境领域的热点问题之一,现有的研究工作聚焦于MPs的检测方法、环境分布、生物毒性等方面,但有关微塑料浸出液的相关研究非常有限,应给予重视。本文通过查阅近年相关的研究论文,就国内外水环境中微塑料浸出液的来源、成分和环境效应等方面的研究进展综述如下。
1 微塑料浸出液除了部分有机活性添加剂,如某些阻燃剂与塑料分子聚合,大部分塑料添加剂与聚合物不发生化学结合,而彼此保持相对独立的化学性质。随着时间推移,塑料在复杂的环境过程中不断老化(如紫外线辐射、物理磨损、化学氧化和生物降解等),不仅改变了塑料的表面性质,还加速了添加剂的释放[14]。因此,无论是塑料单体、低聚物、添加剂和其他聚合杂质(如副产品、催化剂残留物等)都可能从塑料制品中浸出到周围环境和接触介质中,对环境和生物造成危害[15],这些物质统称为塑料浸出液[13]。由于微塑料具有粒径小、比表面积大等特点,使得微塑料更易于释放更多的潜在有害物质。微塑料可通过污水排放、地表径流、大气沉降、水产养殖等[8-10]多种途径进入水环境,因此微塑料添加剂也在多种水生生态系统中检出。调查表明,微塑料在饮用水、淡水和海水环境中都能释放添加剂[16],其成分主要包含PAEs、BPA、溴化阻燃剂(Brominated Flame Retardants, BFRs)等[10, 17-18]。微塑料在环境中停留的时间越长,添加剂浸出量则会越多,导致具有生物放大效应的浸出物在食物链中不断积累,威胁生物健康[19-20](见表 1)。
|
|
表 1 不同的实验条件下微塑料浸出液的特点 Table 1 Characteristics of microplastic leachate under different experimental conditions |
研究表明不同种类的微塑料浸出液的化学成分差别很大,在不同环境中同种微塑料的浸出液成分也不完全相同。微塑料浸出液的成分主要包括聚合物中残存的未反应的单体、低聚物及其降解产物和添加剂。
塑料制品是由单体聚合而成的,聚合物中残存的未反应的单体、低聚物及其降解产物会向水环境中迁移,检测频率较高的单体、低聚物或降解产物为C-11到C-31不等链长的烷烃,这些物质大多数有毒且可被生物吸收[25, 30]。如聚碳酸酯和环氧树脂中未反应原料BPA的含量通常决定了聚合物的毒性程度,BPA能够从塑料制品中浸出,具有致癌性,对人体产生生殖和发育毒性[31];聚苯乙烯(Polystyrene, PS)可浸出致癌物聚乙烯单体和苯,可能会导致生物神经功能紊乱[32];PVC制品在50 ℃以上时会析出氯化氢,对人体造成急性毒性;丙烯腈(Acrylonitrile, ABS)单体是强致癌物质[33];聚对苯二甲酸乙二醇酯(Polyethylene glycol terephthalate, PET)会浸出低聚环化物,从二聚体到五聚体不等[34];尼龙食品包装可浸出己内酰胺(残留前体单体)和低聚物,导致大鼠和兔子产生发育和生殖毒性[35-36]。
为使塑料具有一些特殊的物理性质(如可塑性、延展性、稳定性、阻燃性等),确保在温度、压力、光照等条件下的使用寿命,或避免不需要的性能,在塑料制造过程中会添加数百种不同的添加剂[37]。塑料添加剂根据其功能和结构可以分为四大类:功能添加剂、着色剂、填料及稳定剂。功能添加剂主要包括增塑剂、抗氧化剂、阻燃剂、抗静电剂、润滑剂等,可以改善塑料的物理化学性质;着色剂主要包括颜料、偶氮染料及金属,广泛用于纺织行业;填料主要包括云母、滑石、粘土、碳酸盐等,改善塑料涂层性能的物质;稳定剂主要包括光稳定剂和热稳定剂,例如铅盐、有机锡、锌/钙络合物等,可以避免塑料产品在使用时发生快速降解[38]。在各类添加剂中,最常用的是增塑剂、阻燃剂、抗氧化剂和光热稳定剂[10]。
1.1.1 增塑剂增塑剂通过增强聚合物基质分子键的移动性,降低其结晶性的方式增加塑料的可塑性和柔软度,改善聚合物基质的性质,使塑料更易成型。增塑剂以游离态在塑料制品中存在,广泛应用于食品包装、医疗器械、农用品中,达到降低成本,提高收益的效果。增塑剂的种类繁多,目前商品化的增塑剂有500多种,主要包括邻苯二甲酸酯、脂肪族二元酸酯、脂肪酸酯、苯多酸酯、多元醇酯、环氧烃类等[39],其中邻苯二甲酸酯类增塑剂的生产和消费最大,约占70%[40]。
邻苯二甲酸酯类增塑剂(PAEs)是最常用的增塑剂,被广泛应用在塑料、农膜、化妆品等日用品中,约占增塑剂总产量的80%,在塑料制品中所占质量比例较高,在PVC中甚至达到60%。在常温下PAEs呈无色透明的油状液体,沸点高,挥发性低,具有亲脂性,易溶于有机溶剂,且辛醇-水系数随碳链长度的增加而增加[41]。通常PAEs不与聚合物基质化学结合,在制造、使用和废弃过程中很容易浸出到环境中,目前在空气、天然水体、排放废水、土壤等环境中广泛检测到PAEs[42-43]。PAEs能够通过呼吸道、消化道和皮肤进入人体并在体内积累,部分PAEs如邻苯二甲酸二乙基己酯(DEHP)、邻苯二甲酸二异丁酯(DIBP)、邻苯二甲酸二正丁酯(DnBP)具有致癌性、内分泌毒性、睾丸毒性、卵巢毒性和神经毒性效应[44],被多个国家列为管制化学品[45]。由于PAEs的使用受到限制,对苯二甲酸二辛酯(DOTP)、二乙基羟胺(DEHA)、硬脂酸丁酯、乙酰柠檬酸三丁酯(ATBC)、环己烷二羧酸二异壬基酯(DINCH)、偏苯三酸三辛酯(TOTM)等新型增塑剂逐渐研发投产,随后在水体和沉积物中的浓度逐渐升高[46-47]。虽然新型增塑剂已获得FDA批准作为安全无毒增塑剂,但其仍然可能产生内分泌干扰作用,引起生物的细胞毒性、肝毒性等不良影响[48-51],需要引起重视。
双酚A在工业上是合成聚碳酸酯、环氧树脂、聚砜树脂等多种高分子材料的主要原料,在PC中质量占比可达到65%,在环氧树脂中体积占比可达到30%,也可作为阻燃剂、增塑剂、抗氧化剂、热稳定剂等精细化工的生产原料[52-54]。BPA主要来源为污水废水排放、塑料制品浸出以及PC、PVC等塑料制品降解释放,主要汇集于河流、湖泊以及沉积物,在垃圾渗滤液和沉积物中的浓度通常小于17.2 mg/L,在水样中的浓度通常小于21 μg/L[55]。环境浓度的BPA能够对大鼠产生内分泌干扰作用和致畸作用,能永久性的改变性器官结构并引起肝脏等正常组织的变化[33]。
1.1.2 阻燃剂阻燃剂通过提高聚合物的点火温度、降低燃烧和火焰蔓延的速度来预防火灾,主要可分为卤素基、磷基、氮基和无机盐[56]。溴化阻燃剂(BFRs)是阻燃剂的主要类型之一,在丙烯腈丁二烯苯乙烯(Acrylonitrile Butadiene Styrene, ABS)、高抗冲聚苯乙烯和酚醛树脂等塑料制品中被广泛应用[10]。研究表明,BFRs能够从塑料制品中浸出[57],其中大部分物质具有持久性、生物蓄积性和内分泌干扰作用,产生抗雌激素、抗雄激素、抗孕激素及抗T3-拮抗作用,导致生物产生甲状腺疾病、生殖系统疾病、免疫疾病和癌症等[58]。近年来欧盟、美国、中国等国家已经禁止或限制多种BFRs的使用[10],如:八溴二苯醚、五溴二苯醚、十溴二苯醚和六溴环十二烷。新型溴化阻燃剂(Novel Brominated Flame Retardants, NBFRs)作为替代品应运而生,然而相似的卤代结构使它们的理化性质与传统BFR类似,具有疏水性、亲脂性、半挥发性及较高的辛醇-水分配系数,在多种环境中也能够浸出[59]。目前NBFRs已在水体、沉积物、土壤、生物甚至人类体内检出[60-63],在水生生物的肌肉和内脏中大量积累并随食物链传递,使生物产生氧化应激和内分泌紊乱,并具有潜在神经毒性,对水生生态系统和人类健康造成威胁[59]。因此,NBFRs作为微塑料浸出液的成分之一,在水环境中的生物积累、归宿以及健康风险应给予关注。
1.1.3 抗氧化剂抗氧化剂种类繁多,包括胺、受阻酚类、有机磷类、硫酯类等,主要用于延缓塑料在紫外线照射下的氧化降解[56]。几乎所有的塑料制品都含有抗氧化剂,添加含量可达到20 mg/g,其中聚乙烯(Polyethylene, PE)和聚丙烯(Polypropylene, PP)的抗氧化剂需求量占全球需求量的60%[64-65]。常见的抗氧化剂包括壬基酚(NP)、丁基羟基茴香醚(BHA)、2, 4-二叔丁基苯酚(2, 4-DTBP)、3-(3, 5-二叔丁基-4-羟基苯基)丙酸正十八烷醇酯(Irganox 1076)、2, 6-二叔丁基对甲酚(BHT)、亚磷酸三(2, 4-二叔丁苯基)酯(Irgafos 168)等[64]。其中,壬基酚被广泛用于生产PET和高密度聚乙烯(High Density Polyethylene, HDPE),部分PVC中也有添加[65],对生物体和人类健康造成威胁;Irganox 1076在聚烯烃中的溶解度较低,一般小于1%,容易从聚合物中浸出[66],在塑料中部分Irganox会降解产生BHT和2, 4-DTBP,BHT在未使用塑料和塑料碎片中均有检出,2, 4-DTBP对新生大鼠和水生生物具有毒性效应,且不易生物降解[67-68]。
1.1.4 稳定剂热稳定剂用于防止聚合物在高温下发生热解,通常PVC、聚偏二氯乙烯、氯乙烯共聚物等聚合物需要添加热稳定剂[69]。按照化学类别进行分类,可以将热稳定剂分为主热稳定剂、辅助热稳定剂及二者的混合稳定剂。主热稳定剂主要为无机物和金属有机化合物,如:混合金属盐、有机锡化合物和铅化合物,辅助热稳定剂则为有机物,包括烷基有机亚磷酸酯,环氧化合物和β-二酮[70],通常塑料中会添加铅、锌、锡、钡等重金属作为稳定剂[71],其中金属也能作为着色剂和填料使用。研究表明,PVC材质的手套和玩具浸出液中含有钙、锌、锰、钒、锑、镉、钠等金属,锌含量达到(1 446±78) μg/L[22, 26, 72];PS浸出液中含有钙、铜、锌、钛等金属[71];PE和PP中含有铬、铅、钙、镁等金属,PE中Ca2+和Mg2+含量分别为6 823和429 μg/L,Cd2+含量为6.9 μg/L,约为规定限值的1.7倍[25, 73]。回收PET材料中含有铬、铅、镍等重金属催化剂,最常见的是锑[74]。这些常用的铅、锡、镉、锌/钙络合物等稳定剂对水生生物具有毒性效应并与金属含量呈正相关,不同金属之间还能产生协同作用进一步增强毒性[21, 25]。
光稳定剂,也称紫外线稳定剂(吸收剂),可以保护塑料制品在加工和使用过程中免受紫外线辐射损伤导致的变色、变脆、表面开裂等问题,延长塑料制品的使用寿命。紫外线稳定剂包括吸收紫外线的有机化学物质和散射与反射紫外线的无机锌、二氧化钛颗粒等无机物质两类,通常二者结合使用,后者主要为纳米颗粒[75]。聚烯烃通常含有受阻胺光稳定剂,如Tinuvin622、Tinuvin765和Chimasorb944等。苯并三唑紫外稳定剂(Benzotriazole UV Stabilizers, BUVSS)及其衍生物具有紫外吸收、光散射和光反射三重作用,成为PP、PE等塑料制品最常用的一类有机紫外线稳定剂之一,主要用于建筑、汽车和消费品[76]。BUVSS具有致突变性、持久性、生物蓄积性和内分泌干扰作用[77],不仅在塑料瓶盖、食品包装和购物袋等日常使用的塑料制品中检出,在水体、沉积物、鱼类、土壤等环境中均有检出[78]。在夏威夷考艾岛上搁浅微塑料的浸出液中检出UV-326、UV-328、UV-327、BP-12等紫外线稳定剂,含量高达1 130 μg/g[79]。
1.1.5 其他添加剂润滑剂在分子水平上为聚合物提供润滑作用,降低聚合物的摩擦系数、附着力和粘度。常用的润滑剂有脂肪酸酰胺、脂肪酸酯、金属硬脂酸盐和蜡。如油酸酰胺、芥酸酰胺、硬脂酰胺等[80-81]。塑料制品中检出量较高的润滑剂是正十六烷酸(棕榈酸)和油酸,甘油三酸酯、肉豆腐酸异丙酯、1-二十烷醇、2-己基癸醇、十八烷酰胺、4-甲基苯磺酰胺、1-六烷醇、癸二酸、双(2-乙基己基)酯等润滑剂检出率较低[65]。
开口剂主要为滑石粉、高岭土、二氧化硅、云母等,加入开口剂能使塑料薄膜表面变粗糙,膜间距增大,易于揭开薄膜;爽滑剂也被称为有机开口剂,主要为长链脂肪酸酰胺类,如油酸酰胺、芥酸酰胺等;抗静电剂能有效降低塑料薄膜的静电电位,改善塑料薄膜性能[82]。
1.2 影响微塑料添加剂浸出的主要因素微塑料添加剂浸出液受聚合物自身性质(如聚合物的结构、分子间隙、理化性质、老化程度等)、添加剂性质(如种类、添加量等)和环境因素(如盐度、温度、pH、老化时间等)的影响[70, 83],同一聚合物制成的不同塑料制品浸出液可能产生不同的毒性效应。
1.2.1 聚合物基质聚合物基质由结晶区(定形区)和非结晶区(无定形区)组成,结晶区是指聚合物链有规律地排列在晶格中的分子或分子片段,非结晶区为随机排列的分子,呈现出一种松散的结构,内部结构为玻璃状或橡胶状[84]。单个添加剂分子在聚合物内部晶格中的迁移遵循菲克扩散定律,聚合物的结晶度越高,结构就越稳定和牢固,越不容易扩散及浸出物质,同时,结晶度低的聚合物能够结合更多的疏水有机污染物[85]。聚合物本身具有三维多孔结构,添加剂分散在其分子间隙中,小分子量的添加剂(如聚合物单体和残留溶剂)更容易从分子间隙中浸出,与分子间隙大小匹配的添加剂则不容易从聚合物中浸出[69]。如果聚合物基质发生溶胀,则会使聚合物链松解,空间位阻减小,促进添加剂的扩散[86]。聚合物基质的性质包括聚合物的种类、结构、分子间隙、降解程度、形状、粒径、密度等。
不同聚合物基质浸出液的成分、降解途径、释放速率、浓度和毒性效应均不相同。不同聚合物有不同的降解途径,PE和PP等碳-碳骨架的聚合物易受光氧化和热氧化的影响,通常发生端链断裂降解,主要产物为烯烃、醛和酮,主链上含有杂原子的塑料聚合物易受水解作用的影响,如PE、PET降解产生含有氧化端基的单体和聚合物[37, 87];相同条件下淀粉共混聚合物比LDPE的降解速度更快[88];PS浸出液中溶解有机碳(Dissolved Organic Carbon, DOC)浓度是PVC浸出液的两倍,推测是由于PS中的苯环结构在紫外照射后形成自由基,促进DOC浸出[27];含有发色芳香官能团的聚合物在海洋表层会优先被光降解,如发泡聚苯乙烯(EPS)光降解速率与其他材质的微塑料相比(如PE)更快[89-90]。PVC浸出液浓度、重金属含量比PP、HDPE材质更高[26, 73],对蓝细菌的影响大于HDPE浸出液[91],PP、PS、PE浸出液对大型溞和植物的毒性效应也不同[25]。
微塑料老化时间越长,添加剂的迁移能力越强、微塑料浸出液浓度越大、对生物的毒性效应越强[27-28, 76]。老化PE微塑料浸出液中铬和铅的释放速度约为原始塑料的3倍,浸出量为原始塑料的2~4倍[22];老化程度高的二氧化钛低密度聚乙烯浸出二氧化钛更快,粒径分布更均匀,且浸出量显著高于未老化颗粒[92];橡胶和PVC中的PAHs和PAEs浸出量随时间推移而增加[93]。此外,由于表面化学物质的浸出速度快于聚合物内部的扩散,因此表层聚合物的降解程度与微塑料浸出液成分高度相关,表层发生的结构或化学变化会导致添加剂加速浸出[79]。
微塑料的形状、粒径和密度等物理性质也影响其浸出液的性质。微塑料可以呈现多种形状,如球状、纤维状、薄膜状、不规则形状等,其粒径越小、比表面积越大、与水环境接触的面积就越多,使得其浸出过程更快,如纤维状微塑料的浸出量大于规则形状的微塑料[94]。回收搁浅微塑料进行浸出实验发现,与大粒径微塑料相比,小粒径微塑料浸出液中添加剂的浓度较低、种类较少,这可能是由于小粒径微塑料在环境过程中添加剂的损失量更高,浸出更多,而大碎片中的添加剂能够保存在塑料中,有些塑料碎片浸出液的浓度与原始塑料制品浸出液的浓度相当[79]。微塑料密度会影响其在水体中的位置,低密度聚合物如PP和PE主要分布在表层水中,较高密度的聚合物如PS、PVC则在较深区域占主导地位,因此微塑料承受的压力不同时,其浸出行为会发生改变[95]。
1.2.2 添加剂性质不同添加剂的性质和含量差异会导致相同聚合物基质制成的不同塑料制品产生不同的浸出行为和毒性效应[23, 95]。塑料添加剂的分子量大约在200~2 000 g/mol之间,分子量和体积越小的添加剂分子越容易从聚合物中浸出,例如甲醛、氯乙烯、乙烯、丁二烯等单体及BPA、DEHP等添加剂具有快速迁移的能力[27],分子量较大的有机添加剂则与聚合物基质的结合较紧密,不易从塑料间隙中浸出[56]。如PE、PVC等材料中通常会添加0.1%~27%的环氧化合物,环氧化合物的分子质量越小则毒性效应越强[85];相对分子质量较大的PAEs由于其疏水性和较高的分配系数具有更强的抗迁移能力[23];PVC由于添加剂含量高于PS,浸出液浓度也较高[27]。此外,一些可降解添加剂不仅能够从塑料中浸出,而且可以加速和促进有毒物质从聚合物基质中释放[23, 95]。
某些添加剂能够改变聚合物的结构。如氯原子能够增加PVC的极性,使部分聚氯乙烯链之间产生吸引力,增加PVC的内聚密度,减少自由体积,使添加剂不易向环境浸出[96];含有金属盐或金属氧化物(如氧化铁和氧化锌等)的促降解添加剂能够通过与氧反应改变聚合物基质的结构[97]。氧气进入聚合物基质能够产生含氧羧基、酯、醛和酮等官能团,使聚合物更加亲水,在塑料使用过程中促进塑料光降解[98]。
1.2.3 环境因素多种环境因素(如光照、温度、波浪、盐度、pH等)会使聚合物和添加剂性质发生改变,引起塑料制品的破碎和老化,老化后的微塑料颗粒表面出现裂纹、裂缝,与水环境的接触面积增大,导致微塑料聚合物基质溶胀,空间位阻减小,聚合物基质的结构完整性被破坏并暴露出新的表面,聚合物内部的物质会继续向外部迁移浸出[69],进而增加添加剂的浸出量[57, 99]。
紫外线和温度是引起微塑料降解老化的最主要原因。光降解会改变聚合物的结构和力学性能,使聚合物链断裂降解,表面出现裂纹,暴露内部聚合物,增加微塑料有效表面积;分子量降低,机械稳定性下降,结晶度增加;产生大量含氧官能团,增加表面极性,改变添加剂在水相和聚合物基质间的分配系数,增加亲水性,促进浸出行为[15, 35-36, 79, 90, 99-105]。温度改变会导致物质在水相中的溶解度变化,高温会引起聚合物热分解,产生烷烃、烯烃等产物[15]。当水温升高时,PVC添加剂的浸出量增加[87],PVC、PP、PE、PS等微塑料浸出液中PAEs含量和温度呈正相关[93]。
由于水体中盐度、pH、溶解氧、温度、DOC、压力等因素的差异,不同水体中浸出液的释放速率不同。与实验室配制的溶液相比,天然水体中荧光添加剂浸出量较低,其中海水中浸出量最大,其次是湖水、湿地和河流[38]。盐离子浓度越高,络合作用越强,进而影响聚合物的聚集状态[38, 88],水体盐度越高,PE浸出液中铬和铅的浸出量越高[22]。pH能够影响微塑料浸出过程和毒性效应,在pH较低的条件下,微塑料浸出量增加,金属更易发生解析,在pH较高的条件下,微塑料会携带净负电荷,减弱对污染物的吸附能力[85]。如pH为3时PE浸出液中铅的浸出量远大于pH为7的浸出量,而pH为10时未检测到任何铅[22];pH为5时相比于pH为7~9时,PVC中铅和锡的浸出量显著增大[85]。海水中溶解氧含量低于淡水,溶解氧含量增加能够增强聚合物的表面极性(如羰基指数增加),降低聚合物对疏水污染物的亲和力,导致化学浸出效果增强[15, 25, 69, 88]。DOC含量是影响微塑料浸出的重要因素。高浓度DOC的条件下,DOC会吸附部分不溶性添加剂,加快聚合物浸出速率,显著增加微塑料浸出液中添加剂浓度[69, 106]。例如,DOC可以加快塑料制品中BPA的浸出[57],环境中富里酸和胡敏酸的比例越高,浸出液含量越高[107]。压力条件会影响微塑料的浸出行为,模拟研究表层海水和1 000 m深海水(即0.1和10 MPa)静水压力下塑料中添加剂的浸出过程,结果表明高压条件下分子量大、疏水性强的有机添加剂的浸出被抑制,表层海水中微塑料添加剂的浸出量更大[108]。此外,微生物也能够影响微塑料添加剂的浸出,如海洋原核生物能够促进塑料中PAEs、OPEs和BPS的释放[108],微生物存在会使PVC电缆浸出更多PAEs[100]。
2 微塑料浸出液的提取及检测方法塑料制品在生产过程中添加了大量无机和有机化合物,大多数添加剂的种类与浓度都是未知的,通常无法准确获得用于塑料制品的添加剂信息,因此微塑料浸出液的成分检测较为困难,存在分析的物质不全面或物质浓度低于检出限等问题,可能导致浸出液的毒性被低估。目前国内外关于微塑料浸出液成分的研究很多,但并没有颁布检测塑料浸出液成分的国际或国家标准方法。近年来科研工作者尝试多种微塑料浸出液的提取和检测方法,为准确探究微塑料浸出液的成分提供了重要依据。
2.1 微塑料浸出液的提取方法由于塑料添加剂浸出浓度较小,因此在检测浸出液成分之前需要对样品进行浓缩处理。目前常用的前处理方法有:索氏提取、液液萃取、固相萃取(SPE)、固相微萃取(SPME)、超声提取等方法。
索氏提取是经典的前处理方法,具有高准确度、高重现性,通过利用虹吸原理和溶液回流完成萃取,需要用到索氏提取器,如采用索氏提取测定土壤中6种PAEs[109]。液液萃取的原理是利用不同物质在不同溶剂中的溶解度及分配系数原理进行提取,如采用二氯甲烷提取PP、PE、PET、PS、PVC和EPS等浸出液[25, 64, 110-112];采用正己烷提取PVC及多种塑料制品浸出液中单体及添加剂[30, 40];采用二氯甲烷和正己烷共同提取ABS浸出液[57]等。固相萃取及固相微萃取利用柱层析原理实现目标物质和干扰物质的分离,再将待测物洗脱,如采用SPE柱固相萃取,甲醇洗脱提取14种塑料浸出液成分[14],正己烷和二氯甲烷洗脱提取法国地表水中的有机磷酸酯、PAEs和双酚类物质[18],甲醇乙腈洗脱提取25种塑料制品中PAEs[40],二氯甲烷和甲醇洗脱提取葡萄酒中的PAEs[113];采用HLB柱固相萃取,甲醇洗脱提取PC浸出液[28],乙酸乙酯洗脱提取PVC和PE浸出液中的PAEs[100],甲醇和乙酸乙酯洗脱提取原始塑料的降解产物[87];采用固相微萃取PS浸出液[24]等。超声波提取能够利用超声波的扰动作用使被测物质更易被提取剂提取,如超声震荡提取LDPE浸出液中的二氧化钛[92],超声提取食品接触材料中的5种酸胺类物质[112]等;多数情况作为辅助液液萃取的方法,如液液萃取结合超声波提取PP、PE、EPS、ABS等多种塑料制品浸出液[40, 57, 64, 111]等。
2.2 微塑料浸出液的检测方法对于未知塑料聚合物及单体主要使用光谱法进行检测,最常用的方法是傅里叶变换红外光谱(Fourier Transform Infrared Spectroscopy, FT-IR)和拉曼光谱(Raman Spectroscopy)(见表 2)。傅里叶变换红外光谱技术的辐射的穿透深度只有几微米,因此主要研究微塑料的表面降解,其能够根据样品中特定的化学键类型的吸收频率,将目标聚合物的光谱与标准数据库进行比对,确认化合物基质的类型及降解产物、添加剂等[114]。例如使用FT-IR检测老化和未暴露的聚氯乙烯样品中的碳酸钙[71];使用衰减全反射模式分析LDPE中的抗氧化剂[66]等。拉曼光谱能够得到聚合物的类型和晶体结构的信息,如利用拉曼光谱表征对聚氨酯泡沫(PUF)浸出液、HDPE浸出液的化学成分,检出异氰酸酯、芳香环和二氧化钛等成分,并观察到老化微塑料的拉曼光谱峰强度和二氧化钛浓度增大,基线更不稳定[38, 92]。此外,PS聚合物、BPA和DEHP等含有芳香结构的聚合物和添加剂具有吸光作用,可以通过紫外可见光和荧光光谱检测。采用激发-发射矩阵光谱检测不同光照条件下PVC、PS、BPA、DEHP、PC浸出的溶解有机碳,在浸出液中检出羰基、羟基、羧基、苯酚类似物和类腐殖质成分[27-28]。
|
|
表 2 微塑料浸出液常见成分的检测方法 Table 2 Analytical methods for common components of microplastic leachate |
对于添加的金属成分,一般使用基于原子质量的方法进行检测,如电感耦合等离子体质谱(ICP-MS)、火焰原子吸收分光光度计等方法[14, 73](见表 2)。目前最常使用ICP-MS对浸出液中的金属成分进行分析[25],如在PET浸出液中检出锑、镉、铬、铅、镍等重金属[74];在PVC浸出液中检出锌、铅、钡、锶、铜、镉、铁、锰[114];在HDPE浸出液中检出锌、锰和镍[91]。ICP-MS还可以结合瞬态信号检测到PVC浸出液中的锌、锰、铅、钛等金属[21]。
对于有机物,常采用非靶向的气相色谱-质谱(GC-MS)、液相色谱-质谱(LC-MS)等方法进行检测[38, 91](见表 2)。常采用GC-MS检测邻苯二甲酸酯类增塑剂、抗氧化剂、稳定剂、酸胺类物质、BPA等添加剂,如采用GC-MS检测25种塑料制品浸出液中的PAEs[40];食品接触材料中的5种酸胺类物质[115];PE、PET、PP、PS和PVC浸出液,均有添加剂或者低聚物检出[25, 64, 112],其中PAEs的检出率较高,包括DBP、DiNP、DBP、DnBP、DMP和DEP等[24, 94, 110]。
液相色谱-质谱法有快速、高通量、灵敏性高的特点[116],广泛用于检测聚烯烃材料中苯乙烯单体、BPA、PET低聚物和各类添加剂[30](见表 2)。例如,应用LC-MS分别在HDPE和PVC浸出液中检出5 877和10 658种组分[91];定量检测土壤中6种PAEs、六溴环十二烷、BPA、UV326[109, 111]等。采用高分辨质谱检测PC浸出液中低聚物及其光降解产物[28]及多种原始塑料的降解产物,检出PE、PP浸出液中的二元酸及其同源产物,PS浸出液中PS单体的同源产物及其氧化衍生物,PET浸出液中带有羧酸基团的聚合物碎片[87]。
3 微塑料浸出液对水体环境及水生生物的影响 3.1 微塑料浸出液对水体碳循环的影响海洋中溶解有机碳(Dissolved Organic Carbon, DOC)的来源主要包括大气二氧化碳及陆源有机物的输入、海洋浮游生物的光合作用、生物体的裂解释放及排泄、颗粒物溶解等;移除途径主要包括异养生物摄食、光照分解、颗粒物吸附等[90],因此DOC能反映出水体被有机物污染以及水体中生命活动的情况。
微塑料浸出液包含大量DOC[27, 92],不同聚合物浸出的DOC含量不同,产生DOC的速率和动力学也有差异。根据拟合的动力学曲线,最终约44%的物质浸出为DOC,模拟光照条件下EPS、PP、PE分别在0.3、0.3、0.5年内转化为DOC[90]。据估计,全球海洋中的塑料每年可释放DOC近2.36万t,在室内实验中,PP和PE浸出的DOC在5天内被细菌迅速利用了60%[29],其余无法被微生物利用的DOC,将在海洋中长期存在并积累。虽然塑料光降解生产的DOC通量比海洋中的天然DOC小,但仍可能会导致局部DOC浓度过高,改变DOC水体分布和化学形式,干扰水生生物和微生物群落的活动和组成使其向耐受性强的物种和个体演化,并改变生物对化学物质的敏感性[71, 118]。微塑料也能够吸附环境中的DOC,与微生物利用DOC形成竞争,破坏天然的低营养过程[29]。微塑料浸出液还能够改变周围水环境的pH值。在相同的初始pH条件下,PS浸出液的pH稳定在7左右,HDPE的pH小于7,PVC浸出液的pH均在7.5以上,推测由于PVC中添加了大量的碳酸钙作为填料,释放碳酸盐阴离子形成羟基,导致水体pH升高[71]。
因此,海洋中日益增多的塑料可能会对海洋碳循环、海洋微生物的群落组成、相互作用及多样性等产生影响,潜在影响水生生态系统的结构与功能(见图 1)。
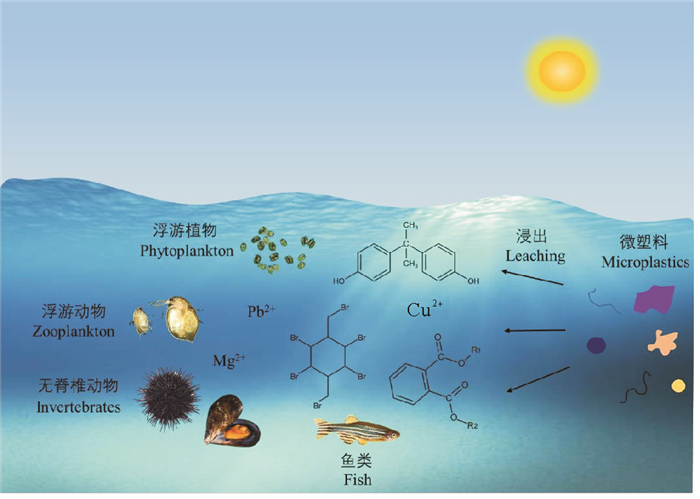
|
图 1 微塑料浸出液对海洋生态系统的影响 Fig. 1 Effects of microplastic leachate on marine ecosystem |
海洋垃圾中80%~85%为塑料垃圾,目前使用最广泛的塑料聚合物种类为聚乙烯、聚苯乙烯、聚氯乙烯、聚丙烯和聚对苯二甲酸乙二酯,总计共占全球塑料总产量的90%[11]。为获得良好的性能,不同塑料制品会根据材质特性使用相应的添加剂,导致其浸出液的成分有所差异。近年来,有关微塑料浸出液对水生生物的不利影响引起广泛关注,因此本文选择使用量最大的三种塑料材质(聚乙烯、聚苯乙烯和聚氯乙烯),重点归纳其浸出液对水生生物的毒性效应。
3.2.1 PE浸出液PE化学稳定性好,具有良好的耐低温性能,密度较低,主要存在于水体上层[95],但PE对光氧化降解敏感[89],通常会添加更多的金属热稳定剂(钙、镁、镉等金属离子)、抗氧化剂(Irganox 1076、Irganox1010、2, 4-DTBP、BHT等)和紫外线稳定剂(UV320、UV326、UV327、UV328等)[25, 32, 64-65]。因此,PE浸出液中的DOC具有高耐光性,能够抑制微生物生长,生物可降解性低,能够在水环境中积累,不稳定的DOC分布可能改变水生生物的群落组成[90]。
PE浸出液中主要的PAEs成分是DIBP和DnBP,水体中部分异养微生物能够在短期内利用这些PAEs迅速增殖,如Pseudomonas azotifigens、Cobetia sp.和红球菌(Rhodococcus rhodochrous)等[23, 119-120],但PAEs、BPA、壬基酚等添加剂的类雌激素作用对水生动植物具有显著负面效应,暴露后大型溞(Daphnia magna)、藤壶(Amphibalanus amphitrite)的死亡率升高,繁殖率和附着率降低,棕色贻贝(Perna perna)和文蛤(Meretrix meretrix)胚胎D形幼虫阶段的死亡率、畸形率升高[25-26, 121-123],珊瑚礁鱼(Pseudochromis fridmani)在48 h内死亡率达到60%,并在体内积累[124]。PE浸出液中的金属离子(如铅、铬、锌、锰、镍等)也会随微塑料的老化过程加速释放[25],抑制铜绿微囊藻(Microcystis aeruginosa)和蓝细菌(Prochlorococcus spp.)的光合作用和细胞生长[22],导致其光合效率降低,产氧量下降[91],明显改变蓝细菌基因的转录水平,而异氰酸酯类化合物会产生络合作用,影响金属的生物可利用性[25]。
3.2.2 PS浸出液PS的单体苯乙烯具有挥发性,对生物有肝脏毒性,能够抑制中枢神经系统,导致生物神经功能紊乱[32, 89]。PS对风化过程敏感性较高,紫外光照下能够释放残留的单体并激发苯环结构形成自由基,使化合键裂解产生低聚物和苯乙烯单体,因此PS浸出液中DOC含量显著高于PE和PVC。
PS浸出液中高浓度的铝离子和钛离子能够与浸出液中的其他成分产生联合毒性效应,长期暴露能够导致大型溞的死亡率显著升高、繁殖率降低、繁殖年龄延后,藤壶死亡率升高,海胆(Lytechinus variegatus)胚胎畸形,畸形率最高达到61.9%[25, 110, 121, 125]。PS浸出液中大量纳米级PS颗粒及PAEs能够在石斑鱼(Epinephelus moara)幼鱼体内积累并加剧PS微塑料颗粒的肝毒性[102]。EPS浸出液中的六溴环十二烷可被贻贝(Mytilus galloprovincialis)吸收,并在体内不断积累[126]。PS浸出液能抑制近头状尖胞藻(Raphidocelis subcapitata)和中肋骨条藻(Skeletonema costatum)的生长[16, 127],但PS浸出液微生物降解率高,能够在短期内显著促进微生物的生长[27, 90],其中的DOC成分也能够增强四种海水藻的光合活性,促进其整体生长[111]。因此PS对部分初级生产者具有一定积极作用。
3.2.3 PVC浸出液PVC在好氧条件下发生自催化的脱氯反应,具有较高的光降解性能,因此很少使用纯PVC制品,通常会加入铅、有机锡等金属光稳定剂[89],并添加大量增塑剂改善其较硬的质地,包括PAEs、多氯联苯、环氧大豆油、乙二酸二乙酯等[32, 65],质量占比可高达60%,对PVC制品浸出液的贡献大于聚合物本身的浸出量,对生物造成威胁[27, 94]。
PVC浸出液组分比PE和PS更为复杂,在剂量敏感性和反应时间方面存在差异。PVC浸出液中铅、锡、镉、镍、锌、锶、铜等重金属含量较高,在紫外光照下浸出量高于FDA限值50倍[73, 95, 106],引起斑马鱼(Danio rerio)胚胎金属结合蛋白(MT2)表达显著上调[117]。邻苯二甲酸二异戊酯、钙锌稳定剂及钠、锡等金属会导致大型溞急性毒性效应,后代体长显著减少[13, 25-26, 110],藤壶死亡率显著上升[121],海胆(Paracentrotus lividus)胚胎发育毒性和致死毒性[21]。PVC浸出液中主要的PAEs成分是DMP和DEP,在光照和微生物降解下PAEs的浸出量会增加5倍,但释放速率较慢。PVC碎片是微生物定殖的良好底物,微生物能够利用PVC浸出液中的PAEs,并促进其浸出[23]。
4 展望塑料的大规模生产导致环境中的微塑料数量不断增加,微塑料浸出液将在水环境中不断积累,对水生生态系统构成严重威胁。本文综述了微塑料浸出液的来源、成分、影响因素、检测方法及其对水环境和水生生物的影响。目前我们对微塑料浸出液的认识还存在一定差距,微塑料浸出液来源、成分、影响因素复杂,对环境的影响也多种多样,目前还没有通用有效的检测方法。对此,未来需要从以下几方面重点开展研究:
(1) 建立微塑料浸出液成分的有效定性、定量和评估方法。微塑料浸出液是一个复杂多样的混合物,虽然已经建立并证明了几种分析方法各有优点,但仍然没有一种高效、快速、低成本、标准的分析方法。目前有关微塑料浸出液的实测数据较少,对微塑料浸出液污染现状的认识还不够,由于缺乏环境浓度的定量数据,很难评估水生生态系统中微塑料浸出液实际暴露条件下造成的生态风险。
(2) 关注微塑料浸出液成分在不同营养级间的食物链传递及生物富集。目前对微塑料浸出液的研究大多停留在个体和种群的毒性效应上,其在生物体内的积累和在食物链、食物网上的传递及其富集有待探究。
(3) 探究环境浓度的微塑料浸出液暴露条件对实验生物的毒性效应。目前大多数研究使用了高浓度、短时间的暴露来研究微塑料浸出液对水生生物的急性毒性效应,缺少低浓度暴露条件下的长期毒性实验数据。但实际环境中不存在大量高浓度的微塑料浸出液成分,需要结合环境中微塑料浸出液污染的现状进行环境剂量及长期暴露实验,进一步揭示其对生物和人类的影响。
(4) 加强塑料制品的生产管理,寻求塑料替代品,并研发塑料回收和微塑料处置技术。塑料生产和使用给人民生活带来了极大的利好,但由此带来的塑料污染问题需要全社会从多角度共同努力方能缓解或解决。首先,政府应出台相关政策,从生产过程加强对塑料制品的规范和监管,逐步减少塑料制品的生产和使用量,并积极寻求塑料替代品,开发以天然聚合物为基础的可生物降解、可再生的添加剂及塑料制品,如聚乙二醇、山梨糖醇、淀粉、果胶、明胶等材料[128]。其次,通过媒体加强对塑料污染和环保意识的宣传,提倡人们“绿色生活”,并注意垃圾分类存放,提高废弃塑料的回收率。第三,加大微塑料污染处置技术的研发, 针对不同功能的水环境生态系统研发有效应对微塑料污染的防治技术方案。
| [1] |
Domenech J, Marcos R. Pathways of human exposure to microplastics, and estimation of the total burden[J]. Current Opinion in Food Science, 2021, 39: 144-151. DOI:10.1016/j.cofs.2021.01.004 (  0) 0) |
| [2] |
Borrelle S, Ringma J, Law K, et al. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution[J]. Science, 2020, 369(6510): 1515-1518. DOI:10.1126/science.aba3656 (  0) 0) |
| [3] |
Thompson R, Olsen Y, Mitchell R, et al. Lost at sea: where is all the plastic?[J]. Science, 2004, 304(5672): 838-838. DOI:10.1126/science.1094559 (  0) 0) |
| [4] |
Amélineau F, Bonnet D, Heitz O, et al. Microplastic pollution in the Greenland Sea: Background levels and selective contamination of planktivorous diving seabirds[J]. Environmental Pollution, 2016, 219: 1131-1139. DOI:10.1016/j.envpol.2016.09.017 (  0) 0) |
| [5] |
Halsband C, Herzke D. Plastic litter in the European Arctic: What do we know?[J]. Emerging Contaminants, 2019, 5: 308-318. DOI:10.1016/j.emcon.2019.11.001 (  0) 0) |
| [6] |
Meng Y, Kelly F, Wright S. Advances and challenges of microplastic pollution in freshwater ecosystems: A UK perspective[J]. Environmental Pollution, 2020, 256: 113445. DOI:10.1016/j.envpol.2019.113445 (  0) 0) |
| [7] |
Rochman C, Kurobe T, Flores I, et al. Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment[J]. Science of the Total Environment, 2014, 493: 656-661. DOI:10.1016/j.scitotenv.2014.06.051 (  0) 0) |
| [8] |
Eerkes-Medrano D, Thompson R, Aldridge D. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs[J]. Water Research, 2015, 75: 63-82. DOI:10.1016/j.watres.2015.02.012 (  0) 0) |
| [9] |
Prata J, Costa J, Lopes I, et al. Environmental exposure to microplastics: An overview on possible human health effects[J]. Science of the Total Environment, 2020, 702: 134455. DOI:10.1016/j.scitotenv.2019.134455 (  0) 0) |
| [10] |
Hermabessiere L, Dehaut A, Paul-Pont I, et al. Occurrence and effects of plastic additives on marine environments and organisms: A review[J]. Chemosphere, 2017, 182: 781-793. DOI:10.1016/j.chemosphere.2017.05.096 (  0) 0) |
| [11] |
叶雪莹. PVC管材源微塑料中邻苯二甲酸酯的释放行为研究[D]. 杭州: 浙江工业大学化学工程学院, 2020. Ye X. The Release Behavior of Phthalates Form PVC Pipe Microplastics[D]. Hangzhou: College of Chemical Engineering, Zhejiang University of Technology, 2020. (  0) 0) |
| [12] |
王旖旎, 林勤保, 钟怀宁, 等. 低密度聚乙烯薄膜中芥酸酰胺的检测及其向食品模拟物的迁移[J]. 中国塑料, 2018, 32(4): 118-124. Wang Y, Lin Q, Zhong H, et al. Detection of erucamide in low-density polyethylene films and its migration to simulant foods[J]. China Plastics, 2018, 32(4): 118-124. (  0) 0) |
| [13] |
Lithner D, Damberg J, Dave G, et al. Leachates from plastic consumer products-screening for toxicity with Daphnia magna[J]. Chemosphere, 2009, 74(9): 1195-1200. DOI:10.1016/j.chemosphere.2008.11.022 (  0) 0) |
| [14] |
Bejgarn S, MacLeod M, Bogdal C, et al. Toxicity of leachate from weathering plastics: An exploratory screening study with Nitocra Spinipes[J]. Chemosphere, 2015, 132: 114-119. DOI:10.1016/j.chemosphere.2015.03.010 (  0) 0) |
| [15] |
Luo H, Zhao Y, Li Y, et al. Aging of microplastics affects their surface properties, thermal decomposition, additives leaching and interactions in simulated fluids[J]. Science of the Total Environment, 2020, 714: 136862. DOI:10.1016/j.scitotenv.2020.136862 (  0) 0) |
| [16] |
Capolupo M, Sørensen L, Jayasena K, et al. Chemical composition and ecotoxicity of plastic and car tire rubber leachates to aquatic organisms[J]. Water Research, 2020, 169: 115270. DOI:10.1016/j.watres.2019.115270 (  0) 0) |
| [17] |
Vandermeersch G, Lourenço H, Alvarez-Muoz D, et al. Environmental contaminants of emerging concern in seafood-European database on contaminant levels[J]. Environmental Research, 2015, 143: 29-45. DOI:10.1016/j.envres.2015.06.011 (  0) 0) |
| [18] |
Schmidt N, Castro-Jiménez J, Fauvelle V, et al. Occurrence of organic plastic additives in surface waters of the Rhône River (France)[J]. Environmental Pollution, 2020, 257: 113637. DOI:10.1016/j.envpol.2019.113637 (  0) 0) |
| [19] |
Faure F, Demars C, Wieser O, et al. Plastic pollution in Swiss surface waters: nature and concentrations, interaction with pollutants[J]. Environmental Chemistry, 2015, 12(5): 582-591. DOI:10.1071/EN14218 (  0) 0) |
| [20] |
Zhang H, Zhou Q, Xie Z, et al. Occurrences of organophosphorus esters and phthalates in the microplastics from the coastal beaches in north China[J]. Science of the Total Environment, 2018, 616-617: 1505-1512. DOI:10.1016/j.scitotenv.2017.10.163 (  0) 0) |
| [21] |
Oliviero M, Tato T, Schiavo S, et al. Leachates of micronized plastic toys provoke embryotoxic effects upon sea urchin Paracentrotus lividus[J]. Environmental Pollution, 2019, 247: 706-715. DOI:10.1016/j.envpol.2019.01.098 (  0) 0) |
| [22] |
Luo H, Li Y, Zhao Y, et al. Effects of accelerated aging on characteristics, leaching, and toxicity of commercial lead chromate pigmented microplastics[J]. Environmental Pollution, 2020, 257: 113475. DOI:10.1016/j.envpol.2019.113475 (  0) 0) |
| [23] |
Paluselli A, Fauvelle V, Galgani F, et al. Phthalate release from plastic fragments and degradation in seawater[J]. Environmental Science and Technology, 2019, 53(1): 166-175. DOI:10.1021/acs.est.8b05083 (  0) 0) |
| [24] |
Wang X, Zheng H, Zhao J, et al. Photodegradation elevated the toxicity of polystyrene microplastics to grouper (Epinephelus moara) through disrupting hepatic lipid homeostasis[J]. Environmental Science and Technology, 2020, 54(10): 6202-6212. DOI:10.1021/acs.est.9b07016 (  0) 0) |
| [25] |
Schiavo S, Oliviero M, Chiavarini S, et al. Adverse effects of oxo-degradable plastic leachates in freshwater environment[J]. Environmental Science and Pollution Research, 2020, 27: 8586-8595. DOI:10.1007/s11356-019-07466-z (  0) 0) |
| [26] |
Lithner D, Nordensvan I, Dave G. Comparative acute toxicity of leachates from plastic products made of polypropylene, polyethylene, PVC, acrylonitrile-butadiene-styrene, and epoxy to Daphnia magna[J]. Environmental Science and Pollution Research, 2012, 19: 1763-1772. DOI:10.1007/s11356-011-0663-5 (  0) 0) |
| [27] |
Lee Y, Murphy K, Hur J. Fluorescence signatures of dissolved organic matter leached from microplastics: Polymers and additives[J]. Environmental Science and Technology, 2020, 54(19): 11905-11914. DOI:10.1021/acs.est.0c00942 (  0) 0) |
| [28] |
Shi Y, Liu P, Wu X, et al. Insight into chain scission and release profiles from photodegradation of polycarbonate microplastics[J]. Water Research, 2021, 195: 116980. DOI:10.1016/j.watres.2021.116980 (  0) 0) |
| [29] |
Romera-Castillo C, Pinto M, Langer T, et al. Dissolved organic carbon leaching from plastics stimulates microbial activity in the ocean[J]. Nature Communications, 2018, 9: 1430. DOI:10.1038/s41467-018-03798-5 (  0) 0) |
| [30] |
Lau O, Wong S. Contamination in food from packaging material[J]. Journal of Chromatography A, 2000, 882(1-2): 255-270. DOI:10.1016/S0021-9673(00)00356-3 (  0) 0) |
| [31] |
Kuvwabo c, Kosarac I, Stewart B, et al. Migration of Bisphenol A from plastic baby bottles, baby bottle liners and reusable polycarbonate drinking bottles[J]. Food Additives and Contaminants: Part A, 2009, 26(6): 928-937. DOI:10.1080/02652030802706725 (  0) 0) |
| [32] |
Arvanitoyannis I, Bosnea L. Migration of substances from food packaging materials to foods[J]. Critical Reviews in Food Science and Nutrition, 2004, 44(2): 63-76. DOI:10.1080/10408690490424621 (  0) 0) |
| [33] |
Howdeshell K, Peterman P, Judy B, et al. Bisphenol A is released from used polycarbonate animal cages into water at room temperature[J]. Environmental Heath Perspectives, 2003, 111(9): 1180-1187. DOI:10.1289/ehp.5993 (  0) 0) |
| [34] |
Begley T, Gay M, Hollifield H. Determination of migrants in and migration from nylon food packaging[J]. Food Additives and Contaminants, 1995, 12(5): 671-676. DOI:10.1080/02652039509374355 (  0) 0) |
| [35] |
Bomfim M, Zamith H, Abrantes S. Migration of ε-caprolactam residues in packaging intended for contact with fatty foods[J]. Food Control, 2011, 22(5): 681-684. DOI:10.1016/j.foodcont.2010.09.017 (  0) 0) |
| [36] |
He Z, Li G, Chen J, et al. Pollution characteristics and health risk assessment of volatile organic compounds emitted from different plastic solid waste recycling workshops[J]. Environment International, 2015, 77: 85-94. DOI:10.1016/j.envint.2015.01.004 (  0) 0) |
| [37] |
Gunaalan K, Fabbri E, Capolupo M. The hidden threat of plastic leachates: A critical review on their impacts on aquatic organisms[J]. Water Research, 2020, 184: 116170. DOI:10.1016/j.watres.2020.116170 (  0) 0) |
| [38] |
Luo H, Xiang Y, He D, et al. Leaching behavior of fluorescent additives from microplastics and the toxicity of leachate to Chlorella vulgaris[J]. Science of the Total Environment, 2019, 678: 1-9. DOI:10.1016/j.scitotenv.2019.04.401 (  0) 0) |
| [39] |
向斌. 食品包装中塑化剂问题解析[J]. 中国包装, 2011(9): 51-53. Xiang B. Analysis of plasticizer problems in food packaging[J]. China Packaging, 2011(9): 51-53. DOI:10.3969/j.issn.1003-062X.2011.09.017 (  0) 0) |
| [40] |
Shen H. Simultaneous screening and determination eight phthalates in plastic products for food use by sonication-assisted extraction/GC-MS methods[J]. Talanta, 2005, 66(3): 734-739. DOI:10.1016/j.talanta.2004.12.021 (  0) 0) |
| [41] |
张霞, 施炎炎, 丁红梅, 等. 塑化剂与食品安全问题探讨[J]. 粮食科技与经济, 2014, 39(1): 44-46. Zhang X, Shi Y, Ding H, et al. Safety problems of plasticizer and food[J]. Grain Science and Technology and Economy, 2014, 39(1): 44-46. DOI:10.3969/j.issn.1007-1458.2014.01.013 (  0) 0) |
| [42] |
Net S, Sempeéreé R, Delmont A, et al. Occurrence, fate, behavior and ecotoxicological state of phthalates in different environmental matrices[J]. Environmental Science and Technology, 2015, 49(7): 4019-4035. DOI:10.1021/es505233b (  0) 0) |
| [43] |
Bergé A, Gasperi J, Rocher V, et al. Phthalates and alkylphenols in industrial and domestic effluents: Case of Paris conurbation (France)[J]. Science of the Total Environment, 2014, 488-489: 26-35. DOI:10.1016/j.scitotenv.2014.04.081 (  0) 0) |
| [44] |
Oehlmann J, Schulte-Oehlmann U, Kloas W, et al. A critical analysis of the biological impacts of plasticizers on wildlife[J]. Biological Sciences, 2009, 364(1526): 2047-2062. DOI:10.1098/rstb.2008.0242 (  0) 0) |
| [45] |
Rowdhwal S, Chen J. Toxic effects of di-2-ethylhexyl phthalate: An overview[J]. Biomed Research International, 2018, 2018: 1750368. (  0) 0) |
| [46] |
Nagorka R, Koschorreck J. Trends for plasticizers in German freshwater environments: Evidence for the substitution of DEHP with emerging phthalate and non-phthalate alternatives[J]. Environmental Pollution, 2020, 262: 114237. DOI:10.1016/j.envpol.2020.114237 (  0) 0) |
| [47] |
Kim Y, Kim S, Liao C, et al. Severe contamination and time trend of legacy and alternative plasticizers in a highly industrialized lake associated with regulations and coastal development[J]. Marine Pollution Bulletin, 2021, 171: 112787. DOI:10.1016/j.marpolbul.2021.112787 (  0) 0) |
| [48] |
Eljezi T, Pinta P, Richard D, et al. In vitro cytotoxic effects of DEHP-alternative plasticizers and their primary metabolites on a L929 cell line[J]. Chemosphere, 2017, 173: 452-459. DOI:10.1016/j.chemosphere.2017.01.026 (  0) 0) |
| [49] |
Sheikh I, Beg M. Structural characterization of potential endocrine disrupting activity of alternate plasticizers di-(2-ethylhexyl) adipate (DEHA), acetyl tributyl citrate (ATBC) and 2, 2, 4-trimethyl 1, 3-pentanediol diisobutyrate (TPIB) with human sex hormone-binding globulin[J]. Reproductive Toxicology, 2019, 83: 46-53. DOI:10.1016/j.reprotox.2018.11.003 (  0) 0) |
| [50] |
Mínguez-Alarcón L, Souter I, Chiu Y, et al. Urinary concentrations of cyclohexane-1, 2-dicarboxyclic acid monohydroxy isononyl ester, a metabolite of the non-phthalate plasticizer di(isononly)cyclohexane-1, 2-dicarboxylate (DINCH), and markers of ovarian response among women attending a fertility center[J]. Environmental Research, 2016, 151: 595-600. DOI:10.1016/j.envres.2016.08.012 (  0) 0) |
| [51] |
Chen X, Ma L, Wang D, et al. Transcriptome profiling and pathway analysis of hepatotoxicity induced by tris (2-ethylhexyl) trimellitate (TOTM) in mice[J]. Environmental Toxicology and Pharmacology, 2016, 41: 62-71. DOI:10.1016/j.etap.2015.11.007 (  0) 0) |
| [52] |
Vilarinho F, Sendón R, van der Kellen A, et al. Bisphenol A in food as a result of its migration from food packaging[J]. Trends in Food Science and Technology, 2019, 91: 33-65. DOI:10.1016/j.tifs.2019.06.012 (  0) 0) |
| [53] |
陈蕾, 高山雪, 徐一卢. 塑料添加剂向生态环境中的释放与迁移研究进展[J]. 生态学报, 2021, 41(8): 3115-3324. Chen L, Gao S, Xu Y. Progress on release and migration of plastic additives to ecological environment[J]. Acta Ecologica Sinica, 2021, 41(8): 3115-3324. (  0) 0) |
| [54] |
Sajiki J, Yonekubo J. Leaching of Bisphenol A (BPA) to seawater from polycarbonate plastic and its degradation by reactive oxygen species[J]. Chemosphere, 2003, 51(1): 55-62. DOI:10.1016/S0045-6535(02)00789-0 (  0) 0) |
| [55] |
Crain D, Eriksen M, Iguchi T, et al. An ecological assessment of Bisphenol-A: Evidence from comparative biology[J]. Reproductive Toxicology, 2007, 24(2): 225-239. DOI:10.1016/j.reprotox.2007.05.008 (  0) 0) |
| [56] |
Hansen E. Hazardous Substances in Plastic Materials[R]. Denmark: The Danish Environmental Protection Agency, 2013.
(  0) 0) |
| [57] |
Sun B, Hu Y, Cheng H, et al. Releases of brominated flame retardants (BFRs) from microplastics in aqueous medium: Kinetics and molecular-size dependence of diffusion[J]. Water Research, 2019, 151: 215-225. DOI:10.1016/j.watres.2018.12.017 (  0) 0) |
| [58] |
Feiteiro J, Mariana M, Cairrão E. Health toxicity effects of brominated flame retardants: From environmental to human exposure[J]. Environmental Pollution, 2021, 285: 117475. DOI:10.1016/j.envpol.2021.117475 (  0) 0) |
| [59] |
Hou R, Lin L, Li H, et al. Occurrence, bioaccumulation, fate, and risk assessment of novel brominated flame retardants (NBFRs) in aquatic environments-A critical review[J]. Water Research, 2021, 198: 117168. DOI:10.1016/j.watres.2021.117168 (  0) 0) |
| [60] |
Jans U. Emerging brominated flame retardants in sediments and soils: A review[J]. Current Pollution Reports, 2016, 2: 213-223. DOI:10.1007/s40726-016-0041-5 (  0) 0) |
| [61] |
Guardia M, Hale R, Harvey E, et al. In situ accumulation of HBCD, PBDEs, and several alternative flame-retardants in the Bivalve (Corbicula fluminea) and Gastropod (Elimia proxima)[J]. Environmental Science and Technology, 2012, 46(11): 5798-5805. DOI:10.1021/es3004238 (  0) 0) |
| [62] |
Chen T, Huang M, Li J. Polybrominated diphenyl ethers and novel brominated flame retardants in human milk from the general population in Beijing, China: Occurrence, temporal trends, nursing infants' exposure and risk assessment[J]. Science of the Total Environment, 2019, 689: 278-286. DOI:10.1016/j.scitotenv.2019.06.442 (  0) 0) |
| [63] |
Guerra P, Eljarrat E, Barceló D. Analysis and occurrence of emerging brominated flame retardants in the Llobregat River basin[J]. Journal of Hydrology, 2010, 383(1-2): 39-43. DOI:10.1016/j.jhydrol.2009.06.052 (  0) 0) |
| [64] |
Rani M, Shim W, Han G, et al. Benzotriazole-type ultraviolet stabilizers and antioxidants in plastic marine debris and their new products[J]. Science of the Total Environment, 2017, 579: 745-754. DOI:10.1016/j.scitotenv.2016.11.033 (  0) 0) |
| [65] |
Loyo-Rosales J, Rosales-Rivera G, Lynch A, et al. Migration of nonylphenol from plastic containers to water and a milk surrogate[J]. Agricultural and Food Chemistry, 2004, 52(7): 2016-2020. DOI:10.1021/jf0345696 (  0) 0) |
| [66] |
Xu A, Roland S, Colin X. Physico-chemical analysis of a silane-grafted polyethylene stabilised with an excess of Irganox 1076Ⓡ. Proposal of a microstructural model[J]. Polymer Degradation and Stability, 2021, 183: 109453. DOI:10.1016/j.polymdegradstab.2020.109453 (  0) 0) |
| [67] |
Hirata-Koizumi M, Hamamura M, Furukawa H, et al. Elevated susceptibility of newborn as compared with young rats to 2-tert-butylphenol and 2, 4-di-tert-butylphenol toxicity[J]. Congenital Anomalies, 2005, 45(4): 146-153. DOI:10.1111/j.1741-4520.2005.00084.x (  0) 0) |
| [68] |
Dopico-García M, López-Vilariíó J, González-Rodríguez M. Antioxidant content of and migration from commercial polyethylene, polypropylene, and polyvinyl chloride packages[J]. Agricultural and Food Chemistry, 2007, 55(8): 3225-3231. DOI:10.1021/jf070102+ (  0) 0) |
| [69] |
Teuten E, Saquling J, Knappe D, et al. Transport and release of chemicals from plastics to the environment and to wildlife[J]. Philosophical Transactions of the Royal Society B Biological Sciences, 2009, 364(1526): 2027-2045. DOI:10.1098/rstb.2008.0284 (  0) 0) |
| [70] |
Hahladakis J, Velis C, Weber R, et al. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling[J]. Journal of Hazardous Materials, 2018, 344: 179-199. DOI:10.1016/j.jhazmat.2017.10.014 (  0) 0) |
| [71] |
Lan T. Contaminant Release from Aged Microplastic[M]. USA: Springer, Cham, 2021: 1-21.
(  0) 0) |
| [72] |
Oliviero M, Tato T, Schiavo S, et al. Leachates of micronized plastic toys provoke embryotoxic effects upon sea urchin Paracentrotus lividus[J]. Environmental Pollution, 2019, 247: 706-715. DOI:10.1016/j.envpol.2019.01.098 (  0) 0) |
| [73] |
Omolaoye J, Uzairu A, Gimba C. Heavy metal assessment of some soft plastic toys imported into Nigeria from China[J]. Journal of Environmental Chemistry and Ecotoxicology, 2010, 2(8): 126-130. (  0) 0) |
| [74] |
Whitt M, Brown W, Danes J, et al. Migration of heavy metals from recycled polyethylene terephthalate during storage and microwave heating[J]. Journal of Plastic Film and Sheeting, 2016, 32(2): 189-207. DOI:10.1177/8756087915590190 (  0) 0) |
| [75] |
Fent K, Kunz P, Gomez E. UV Filters in the aquatic environment induce hormonal effects and affect fertility and reproduction in fish[J]. Chimia International Journal for Chemistry, 2008, 62(5): 368-375. DOI:10.2533/chimia.2008.368 (  0) 0) |
| [76] |
Montesdeoca-Esponda S, Vega-Morales Z T, Sosa-Ferrera Z, et al. Extraction and determination methodologies for benzotriazole UV stabilizers in personal-care products in environmental and biological samples[J]. TrAC Trends in Analytical Chemistry, 2013, 51: 23-32. DOI:10.1016/j.trac.2013.05.012 (  0) 0) |
| [77] |
Kim J, Chang K, Isobe T, et al. Acute toxicity of benzotriazole ultraviolet stabilizers on freshwater crustacean (Daphnia pulex)[J]. The Journal of Toxicological Sciences, 2011, 36(2): 247-251. DOI:10.2131/jts.36.247 (  0) 0) |
| [78] |
Sakuragi Y, Takada H, Sato H, et al. An analytical survey of benzotriazole UV stabilizers in plastic products and their endocrine-disrupting potential via human estrogen and androgen receptors[J]. Science of the Total Environment, 2021, 800: 149374. DOI:10.1016/j.scitotenv.2021.149374 (  0) 0) |
| [79] |
Tanaka K, Takada H, Ikenaka Y, et al. Occurrence and concentrations of chemical additives in plastic fragments on a beach on the island of Kauai, Hawaii[J]. Marine Pollution Bulletin, 2020, 150: 110732. DOI:10.1016/j.marpolbul.2019.110732 (  0) 0) |
| [80] |
Bhunia K, Sablani S, Tang J, et al. Migration of chemical compounds from packaging polymers during microwave, conventional heat treatment, and storage[J]. Comprehensive Reviews in Food Science and Food Safety, 2013, 12(5): 523-545. DOI:10.1111/1541-4337.12028 (  0) 0) |
| [81] |
杨宏伟, 马玉红, 李召良, 等. 塑料润滑剂的发展现状及应用[J]. 广州化工, 2013, 41(2): 20-25. Yang H W, Ma Y H, Li S L, et al. Development and application of plastic lubricants[J]. Guangzhou Chemical Industry, 2013, 41(2): 20-25. DOI:10.3969/j.issn.1001-9677.2013.02.007 (  0) 0) |
| [82] |
任晓兵. 开口爽滑剂在低密度聚乙烯薄膜中的应用[J]. 当代化工, 2017, 46(1): 173-176. Ren X B. Application of anti-blocking and slip additives in LDPE film[J]. Contemporary Chemical Industry, 2017, 46(1): 173-176. DOI:10.3969/j.issn.1671-0460.2017.01.049 (  0) 0) |
| [83] |
Kwan C, Takada H. Release of additives and monomers from plastic wastes[J]. Hazardous Chemicals Associated with Plastics in the Marine Environment, 2016, 78: 51-70. (  0) 0) |
| [84] |
Lau O, Wong S. Contamination in food from packaging material[J]. Journal of Chromatography A, 2000, 882(1-2): 255-270. DOI:10.1016/S0021-9673(00)00356-3 (  0) 0) |
| [85] |
Li J, Zhang K, Zhang H. Adsorption of antibiotics on microplastics[J]. Environmental Pollution, 2018, 237: 460-467. DOI:10.1016/j.envpol.2018.02.050 (  0) 0) |
| [86] |
Lee H, Byun D, Kim J, et al. Desorption modeling of hydrophobic organic chemicals from plastic sheets using experimentally determined diffusion coefficients in plastics[J]. Marine Pollution Bulletin, 2018, 126: 312-317. DOI:10.1016/j.marpolbul.2017.11.032 (  0) 0) |
| [87] |
Gewert B, Plassmann M, Sandblom O, et al. Identification of chain scission products released to water by plastic exposed to ultraviolet light[J]. Environmental Science and Technology, 2018, 5(5): 272-276. (  0) 0) |
| [88] |
Sudhakar M, Trishul A, Doble M, et al. Biofouling and biodegradation of polyolefins in ocean waters[J]. Polymer Degradation and Stability, 2007, 92(9): 1743-1752. DOI:10.1016/j.polymdegradstab.2007.03.029 (  0) 0) |
| [89] |
Gewert B, Plassmann M, MacLeod M. Pathways for degradation of plastic polymers floating in the marine environment[J]. Environmental Science: Processes Impacts, 2015, 17: 1513-1521. DOI:10.1039/C5EM00207A (  0) 0) |
| [90] |
Zhu L, Zhao S, Bittar T, et al. Photochemical dissolution of buoyant microplastics to dissolved organic carbon: Rates and microbial impacts[J]. Journal of Hazardous Materials, 2020, 383: 121065. DOI:10.1016/j.jhazmat.2019.121065 (  0) 0) |
| [91] |
Tetu S G, Sarker I, Schrameyer V, et al. Plastic leachates impair growth and oxygen production in Prochlorococcus, the ocean's most abundant photosynthetic bacteria[J]. Communications Biology, 2019, 2: 184. DOI:10.1038/s42003-019-0410-x (  0) 0) |
| [92] |
Luo H, Xiang Y, Li Y, et al. Weathering alters surface characteristic of TiO2-pigmented microplastics and particle size distribution of TiO2 released into water[J]. Science of the Total Environment, 2020, 729: 139083. DOI:10.1016/j.scitotenv.2020.139083 (  0) 0) |
| [93] |
Kida M, Koszelnik P. Investigation of the presence and possible migration from microplastics of phthalic acid esters and polycyclic aromatic hydrocarbons[J]. Journal of Polymers and the Environment, 2021, 29: 599-611. DOI:10.1007/s10924-020-01899-1 (  0) 0) |
| [94] |
Sarah Y, Bruce T, Bridges W, et al. Responses of Hyalella Azteca to acute and chronic microplastic exposures[J]. Environmental Toxicology and Chemistry, 2015, 34(11): 2564-2572. DOI:10.1002/etc.3093 (  0) 0) |
| [95] |
Erni-Cassola G, Zadjelovic V, Gibson M, et al. Distribution of plastic polymer types in the marine environment; A meta-analysis[J]. Journal of Hazardous Materials, 2019, 369: 691-698. DOI:10.1016/j.jhazmat.2019.02.067 (  0) 0) |
| [96] |
Pascall M, Zabik M, Zabik M, et al. Uptake of polychlorinated biphenyls (PCBs) from an aqueous medium by polyethylene, polyvinyl chloride, and polystyrene films[J]. Agricultural and Food Chemistry, 2005, 53(1): 164-169. DOI:10.1021/jf048978t (  0) 0) |
| [97] |
Shawaphun S, Manangan T, Wacharawichanant S. Thermo- and photo-degradation of LDPE and PP films using metal oxides as catalysts[J]. Advanced Materials Research, 2010, 93-94: 505-508. DOI:10.4028/www.scientific.net/AMR.93-94.505 (  0) 0) |
| [98] |
Wei R, Tiso T, Bertling J, et al. Possibilities and limitations of biotechnological plastic degradation and recycling[J]. Nature Catalysis, 2020, 3: 867-871. DOI:10.1038/s41929-020-00521-w (  0) 0) |
| [99] |
Hordon A, Walton A, Spurgeon D, et al. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities[J]. Science of the Total Environment, 2017, 586: 127-141. DOI:10.1016/j.scitotenv.2017.01.190 (  0) 0) |
| [100] |
Paluselli A, Fauvelle V, Galgani F, et al. Phthalate release from plastic fragments and degradation in seawater[J]. Environmental Science and Technology, 2019, 53(1): 166-175. DOI:10.1021/acs.est.8b05083 (  0) 0) |
| [101] |
Yang Y, Hu C, Zhong H, et al. Effects of ultraviolet (UV) on degradation of Irgafos 168 and migration of its degradation products from polypropylene films[J]. Agricultural and Food Chemistry, 2016, 64(41): 7866-7873. DOI:10.1021/acs.jafc.6b03018 (  0) 0) |
| [102] |
Wang X, Zheng H, Zhao J, et al. Photodegradation elevated the toxicity of polystyrene microplastics to grouper (Epinephelus moara) through disrupting hepatic lipid homeostasis[J]. Environmental Science and Technology, 2020, 54(10): 6202-6212. DOI:10.1021/acs.est.9b07016 (  0) 0) |
| [103] |
Rummel C, Escher B, Sandblom O, et al. Effects of leachates from UV-weathered microplastic in Cell-Based bioassays[J]. Environmental Science and Technology, 2019, 53(15): 9214-9223. DOI:10.1021/acs.est.9b02400 (  0) 0) |
| [104] |
Wang T, Ma Y, Ji R. Aging processes of polyethylene mulch films and preparation of microplastics with environmental characteristics[J]. Bulletin of Environmental Contamination and Toxicology, 2021, 107: 736-740. DOI:10.1007/s00128-020-02975-x (  0) 0) |
| [105] |
Zhu K, Jia H, Sun Y, et al. Long-term phototransformation of microplastics under simulated sunlight irradiation in aquatic environments: Roles of reactive oxygen species[J]. Water Research, 2020, 173: 115564. DOI:10.1016/j.watres.2020.115564 (  0) 0) |
| [106] |
Al-Malack M. Migration of lead from unplasticized polyvinyl chloride pipes[J]. Journal of Hazardous Materials, 2001, 82(3): 263-274. DOI:10.1016/S0304-3894(00)00366-6 (  0) 0) |
| [107] |
Xu S, Zhang H, He P, et al. Leaching behaviour of Bisphenol A from municipal solid waste under landfill environment[J]. Environmental Technology, 2011, 32(11): 1269-1277. DOI:10.1080/09593330.2010.535175 (  0) 0) |
| [108] |
Fauvelle V, Garel M, Tamburini C, et al. Organic additive release from plastic to seawater is lower under deep-sea conditions[J]. Nature Communications, 2021, 12: 4426. DOI:10.1038/s41467-021-24738-w (  0) 0) |
| [109] |
曹攽, 马军, 李云木子, 等. 索氏提取-液相色谱法测定土壤中邻苯二甲酸酯类物质[J]. 地质学刊, 2011, 35(1): 73-77. Cao B, Ma J, Li Y M Z, et al. Determination of phthalic acid esters in soils by soxhlet technology-high precision liquid chromatography[J]. Journal of Geology, 2011, 35(1): 73-77. DOI:10.3969/j.issn.1674-3636.2011.01.73 (  0) 0) |
| [110] |
Schrank I, Trotter B, Dummert J, et al. Effects of microplastic particles and leaching additive on the life history and morphology of Daphnia magna[J]. Environmental Pollution, 2019, 255(2): 113233. (  0) 0) |
| [111] |
Chae Y, Hong S H, An Y. Photosynthesis enhancement in four marine microalgal species exposed to expanded polystyrene leachate[J]. Ecotoxicology and Environmental Safety, 2020, 189: 109936. DOI:10.1016/j.ecoenv.2019.109936 (  0) 0) |
| [112] |
Suhrhoff T, Scholz-Böttcher B. Qualitative Impact of Salinity, UV radiation and turbulence on leaching of organic plastic additives from four common plastics - A lab experiment[J]. Marine Pollution Bulletin, 2016, 102(1): 84-94. DOI:10.1016/j.marpolbul.2015.11.054 (  0) 0) |
| [113] |
Del Carlo M, Pepe A, Sacchetti G, et al. Determination of phthalate esters in wine using solid-phase extraction and gas chromatography-mass spectrometry[J]. Food Chemistry, 2008, 111(3): 771-777. DOI:10.1016/j.foodchem.2008.04.065 (  0) 0) |
| [114] |
Li J, Song Y, Cai Y. Focus topics on microplastics in soil: Analytical methods, occurrence, transport, and ecological risks[J]. Environmental Pollution, 2020, 257: 113570. DOI:10.1016/j.envpol.2019.113570 (  0) 0) |
| [115] |
郭娟娟, 唐熙, 陈丽娟, 等. 超声提取-气相色谱-质谱法同时测定尼龙等食品接触材料中5种酰胺类物质[J]. 理化检验(化学分册), 2020, 56(6): 696-700. Guo J J, Tang X, Chen L J, et al. GC-MS determination of 5 amides in Nylon and other food-contacting materials with separation by ultrasonicated extraction[J]. PTCA(Part B: Chem Anal), 2020, 56(6): 696-700. (  0) 0) |
| [116] |
Li X, Xiong W, Lin H, et al. Analysis of 16 phthalic acid esters in food simulants from plastic food contact materials by LC-ESI-MS/MS[J]. Journal of Separation Science, 2013, 36(3): 477-484. DOI:10.1002/jssc.201200689 (  0) 0) |
| [117] |
Boyle D, Catarino A, Clark N, et al. Polyvinyl chloride (PVC) plastic fragments release Pb additives that are bioavailable in zebrafish[J]. Environmental Pollution, 2020, 263: 114422. DOI:10.1016/j.envpol.2020.114422 (  0) 0) |
| [118] |
Carlson C, Giovannoni S, Hansell D, et al. Effect of nutrient amendments on bacterioplankton production, community structure, and DOC utilization in the northwestern Sargasso Sea[J]. Aquatic Microbial Ecology, 2002, 30: 19-36. DOI:10.3354/ame030019 (  0) 0) |
| [119] |
Fernández-Juárez V, López-Alforja X, Frank-Comas A, et al. "The good, the bad and the double-sword" effects of microplastics and their organic additives in marine bacteria[J]. Front Microbiol, 2020, 11: 581118. (  0) 0) |
| [120] |
Wang J, Zhang M, Chen T, et al. Isolation and identification of a di-(2-Ethylhexyl) phthalate-degrading bacterium and its role in the bioremediation of a contaminated soil[J]. Pedosphere, 2015(2): 202-211. (  0) 0) |
| [121] |
Li H, Getzinger G, Ferguson P, et al. Effects of toxic leachate from commercial plastics on larval survival and settlement of the Barnacle Amphibalanus amphitrite[J]. Environmental Science and Technology, 2015, 50(2): 924-931. (  0) 0) |
| [122] |
Silva P, Nobre C, Resaffe P, et al. Leachate from microplastics impairs larval development in brown mussels[J]. Water Research, 2016, 106: 364-370. DOI:10.1016/j.watres.2016.10.016 (  0) 0) |
| [123] |
Ke A, Chen J, Zhu J, et al. Impacts of leachates from single-use polyethylene plastic bags on the early development of clam Meretrix meretrix (Bivalvia Veneridae)[J]. Marine Pollution Bulletin, 2019, 142: 54-57. DOI:10.1016/j.marpolbul.2019.03.029 (  0) 0) |
| [124] |
Hamlin H, Marciano K, Downs C. Migration of nonylphenol from food-grade plastic is toxic to the coral reef fish species Pseudochromis Fridmani[J]. Chemosphere, 2015, 139: 223-228. DOI:10.1016/j.chemosphere.2015.06.032 (  0) 0) |
| [125] |
Nober C, Santana M, Maluf A, et al. Assessment of microplastic toxicity to embryonic development of the sea urchin Lytechinus variegatus (Echinodermata: Echinoidea)[J]. Marine Pollution Bulletin, 2015, 92(1-2): 99-104. DOI:10.1016/j.marpolbul.2014.12.050 (  0) 0) |
| [126] |
Jang M, Shim W, Han G, et al. Relative importance of aqueous leachate versus particle ingestion as uptake routes for microplastic additives (hexabromocyclododecane) to mussels[J]. Environmental Pollution, 2021, 270: 116272. DOI:10.1016/j.envpol.2020.116272 (  0) 0) |
| [127] |
Simon M, Hartmann N, Vollertsen J. Accelerated weathering increases the release of toxic leachates from microplastic particles as demonstrated through altered toxicity to the green algae Raphidocelis subcapitata[J]. Toxics, 2021, 9(8): 185. DOI:10.3390/toxics9080185 (  0) 0) |
| [128] |
Hazrati K, Sapuan S, Zuhri M, et al. Effect of plasticizers on physical, thermal, and tensile properties of thermoplastic films based on Dioscorea hispida starch[J]. International Journal of Biological Macromolecules, 2021, 185: 219-228. DOI:10.1016/j.ijbiomac.2021.06.099 (  0) 0) |
2. The Key Laboratory of Marine Environment and Ecology, Ministry of Education, Ocean University of China, Qingdao 266100, China
 2022, Vol. 52
2022, Vol. 52

