| 钙钛矿太阳能电池ABX3层研究进展 |
随着社会发展和科技进步,石化能源日益缺乏和环境的恶化,可持续清洁能源的利用成为国内外共同的发展目标。太阳能作为清洁新能源,受到了广泛关注。根据GREEN等人的划分[1-2],可以将太阳能电池划分为三代:以单晶硅为代表的单晶型太阳能电池;以铜铟镓硒化物(CIGS)和碲化镉(CdTe)为代表的薄膜太阳能电池;基于纳米技术以及新兴材料的新型太阳能电池。新型太阳能电池具有成本低、耗能少、环境友好等特点,得到了大家的青睐。其中有机-无机杂化钙钛矿太阳能电池被评论为“光伏领域的明日之星”[3]。
钙钛矿太阳能电池(PSC)可实现低成本的溶液加工、光吸收能力较强[4]、载流子迁移率高[5]、载流子寿命长[6]、可调控带隙等优点[7-11]。短短几年,小面积电池光电转化效率已经由3.81%[12]提高到22.1%[13],模块器件的光电转化效率达到了8.7%[14]。PSC是在染料敏化太阳能电池(DSSC)基础上发展的新一代太阳能电池。REGAN等人[15]通过提高TiO2层的比表面积,获得了7.9%的光电转化效率,随后TiO2形貌的优化成为提高DSSC效率的突破点[16-17]。其中光吸收层材料具有ABX3钙钛矿型的结构(如图 1所示),八面体的[BX6]2-通过和A位的有机阳离子基团(如NHHN3+、CH3NH3+等)间通过离子键作用,形成卤素八面体共顶连接的稳定三维网络结构。
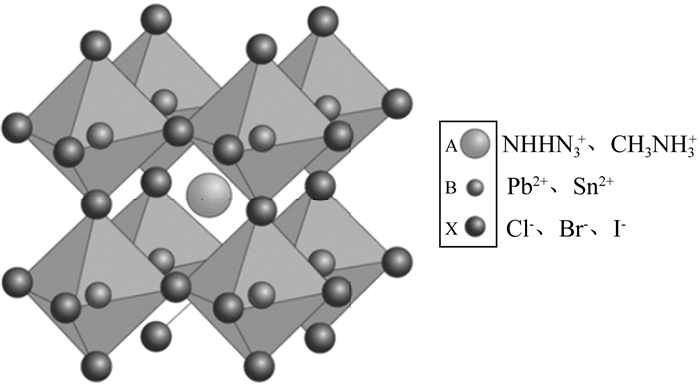 |
| 图 1 ABX3钙钛矿晶体结构示意图[2] |
这种结构的材料具有以下特征:
1) 较好的光电特性,激子束缚能较小[18];
2) 较大的介电常数,电子和空穴可以有效传输并收集[4];
3) 吸收系数高,表现出很好的光吸收[19],光学吸收系数高于104 cm-1[20];
4) 可以同时传输电子和空穴,且传输距离可达到100 nm以上,甚至超过1 μm,光吸收层可以充分吸收太阳光能量[2, 21-24]。
这些特性使其应用在电池器件上表现出较高的开路电压(1.3 V)[20]和短路电流。
1 PSCs的结构现阶段,根据钙钛矿太阳能电池的发展,其构造主要分为介孔结构和平面结构,如图 2所示。
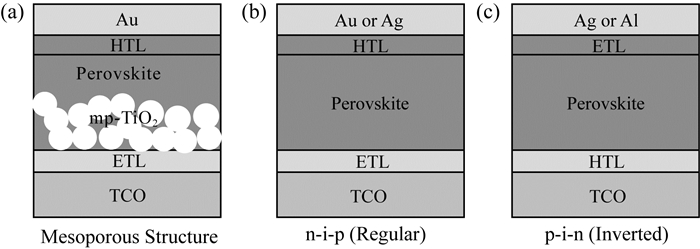 |
| 注:(a)介孔结构;(b)正型平面结构;(c)反型平面结构[25] 图 2 钙钛矿太阳能电池经典结构 |
2009年,KOJIMA等人[12]将CH3NH3PbBr3和CH3NH3PbI3应用到传统的染料敏化太阳能电池中,仅分别获得了3.1%和3.8%的光电转化效率。直到2012年,KIM等人[26]使用固态的空穴传输材料Spiro-OMeTAD来取代液态电解质,使得光电转化效率超过9%。其中TiO2致密层的作用主要是收集电子,阻挡空穴。LEIJTENS等人[27]发现钙钛矿的浓度与TiO2介孔层的厚度在一定的匹配条件下才能形成连续的膜。除了TiO2纳米颗粒外,TiO2纳米线、纳米管、纳米棒以及ZnO、NiOx等都被研究过[8, 28-35]。
LEE等人[33]选用了绝缘的Al2O3作为介孔层制备出结构为FTO/致密层TiO2/Al2O3/钙钛矿/Spiro-OMeTAD/Ag的太阳能电池,光电转化效率与开路电压分别达到了10%和0.98V,与使用TiO2多孔层的钙钛矿电池相比有所提高。在这种结构中,Al2O3多孔层仅仅起到了“骨架”支撑的作用。随后,LIU等人[9]首次使用双源共蒸发法在TiO2致密层上沉积钙钛矿作为光吸收层,这种n-i-p型平面结构的钙钛矿太阳能电池更加类似于OPV结构(如图 2所示)。
2 光吸收层组分的控制通过对其工作机理深入的研究,发现结构的优化以及材料的选择成为提高太阳能电池光电转化效率的关键[36-37]。其中光吸收层起到核心作用,从材料到制备工艺,每个环节都对转化效率产生很大影响。本文主要讨论光吸收层的掺杂对电池的影响。
2.1 卤素掺杂 2.1.1 溴离子掺杂CH3NH3PbX3的电子结构与X的p轨道和Pb的p轨道有关,因此可以通过调整X的p轨道来调整CH3NH3PbX3的带隙[38],从而可以达到对吸收光谱可见光范围全覆盖[39-40]。
CAI等人[41]制备CH3NH3PbBr3沉积在TiO2介孔层上,聚[N-9-癸-2,7-咔唑-alt-3,6-二(5-噻吩)-2,5-二辛基-2,5-二氢吡咯基[3,4]吡咯-1,4-二酮](PCBTDPP)作为空穴传输层,获得了3.04%的效率,开路电压VOC1.15 V。EDRI等人[20]用CH3NH3PbBr3作为光吸收层,Al2O3作为介孔层,N,N′-二烷基苝二酰亚胺(PDI)作为HTM,相应电池的VOC可高达1.3 V。
NOH等人[7]用Br掺杂的CH3NH3Pb(I1-xBrx)3作为光吸收层来改善器件的性能,聚三芳胺(PTAA)作为空穴传输层,制备出结构为FTO/致密层TiO2/介孔层TiO2/CH3NH3Pb(I1-xBrx)3/PTAA/Au的太阳能电池,获得12.3%的光电转化效率。随着掺Br量的提高,x=0时CH3NH3PbI3的四方晶相在x=0.2时转变为立方相。通过对不同Br掺杂量的电池的比较,发现由于晶格常数降低和立方相的转变,一定量的Br掺杂可以提高电荷传输性能和稳定性。同样,Br掺杂在HC(NH2)2PbIyBr3-y体系中也引起晶相转变。EPERON等人[39]使用较大离子半径的甲脒胺基碘化铅(HC(NH2)2+) (FA)来取代A位的甲胺基团CH3NH3+ (MA),获得了1.48 eV带宽的FAPbI3型钙钛矿(吸收起始波长约800 nm)[4]。通过改变Br的含量,晶体结构从立方相(y < 0.5,Br富集)转变为四方相(y>0.7,I富集)。KULKARNI等人[38]使用两步法沉积MAPb(I1-xBrx)3,发现PbI2膜在MABr/MAI的异丙醇混合溶液中的浸泡时间以及两种卤素前驱体的体积比对最终的MAPb(I1-xBrx)3组分起决定性作用。JEON等人[42]将(FAPbI3)1-x(MAPbBr3)x作为光吸收层应用到钙钛矿太阳能电池中,制备结构为FTO/TiO2/钙钛矿层/PTAA/Au的太阳能电池,通过对两种钙钛矿材料比例的控制,随着x的提高电流密度达到22.0 mAcm-2。且当x=0.15时有助于形成连续致密的膜。在此基础上优化电池结构:FTO/致密层TiO2/介孔层TiO2:钙钛矿层/钙钛矿层/PTAA/Au,最终达到了19.0%的转化效率。CAO等人[43]将MAPbI2Br作为光吸收层,用于无空穴传输材料,用于碳电极的电池中,获得了11.03%的光电转化效率。为了方便比较,我们在表 1列出了最近几年钙钛矿光吸收层材料的Br掺杂的性能研究。
| 表 1 钙钛矿太阳能电池光吸收层溴离子掺杂对性能的影响 |
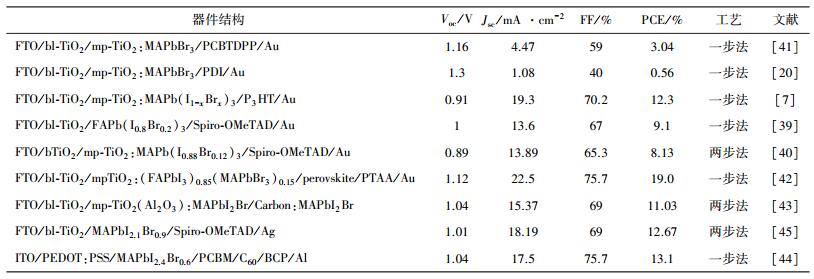 |
在控制钙钛矿结晶的研究上,BI等人[44]使用蒸汽辅助的方法获得了较大的晶粒(如图 3(a)所示),可以贯穿整个钙钛矿层以减少晶界引起的载流子复合。ZHU等人[45]使用了一种快速有效的卤素交换来实现Br掺杂,并通过控制在MABr的异丙醇溶液中浸泡时间来调节Br的掺杂量,获得1.75eV带隙的MAPbI2.1Br0.9型钙钛矿。应用在平面结构中,获得了12.67%的光电转化效率。这种掺杂工艺使晶粒垂直界面生长并且形成致密平整的钙钛矿膜(如图 3(c)所示)。
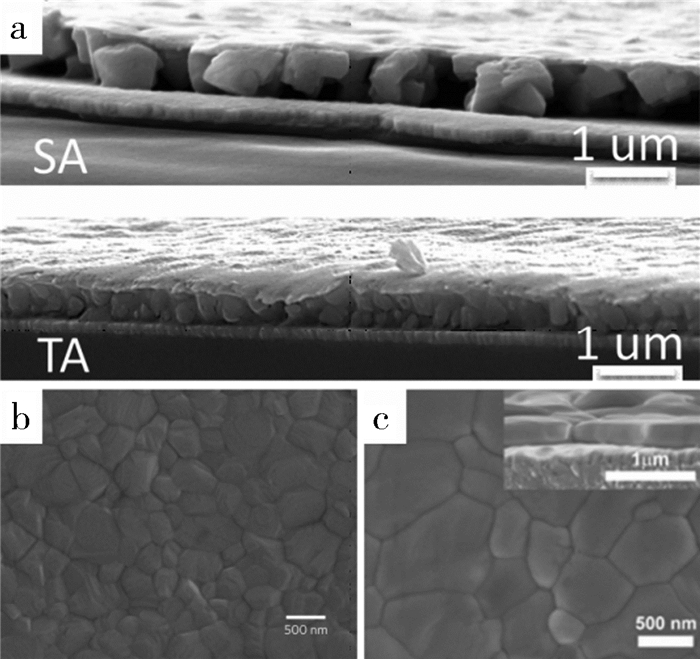 |
| a.分别用溶剂辅助加热(SA)和加热(TA)制备600 nm厚度的MAPbI 2.4Br 0.6钙钛矿层断面SEM图[44];b.钙钛矿层的高分辨SEM图[46];c.卤素交换制备MAPbI2.1Br0.9薄膜的SEM平面图和断面图[47]. 图 3 钙钛矿层的扫描电镜图 |
同样,为了改善晶粒大小及分布均匀度,JEON等人[46]从降低前驱体MAX与PbX2(X=Br、I)之间的反应速率出发,将一定量的二甲基亚砜(DMSO)与γ-丁内酯(GBL)混合作为前驱体溶剂[47],与DMSO相互作用形成CH3NH3I-PbI2-DMSO中间体,能有效减缓两种前驱体间的反应速率,进而有利于生成均匀致密的活性层薄膜(如图 3(b)所示)。图 3对比了几种工艺条件下钙钛矿层的扫描电镜图。
2.1.2 氯离子掺杂2013年,STRANKS等人[5]通过时间分辨光谱研究表明,CH3NH3PbI(3-x)Clx的扩散长度大于1 μm。CONINGS等人[48]通过优化前驱体的浓度,制备正型钙钛矿太阳能电池,获得了10.4%的光电转化效率,其中光电流密度达到20.8 mA·cm-2。
DOCAMPO等人[49]将一定量的MACl添加到MAI溶液中,通过两步法制备正型钙钛矿太阳能电池,发现氯的引入使得钙钛矿的光生电子寿命达到了300 ns,并提高了在起始吸收边的光吸收,使得最终的光电流密度达到了22 mA·cm-2,电池光电转化效率达到了15.41%。
DAR等人[50]使用PbCl2作为添加剂,在两步法的基础上探究了Cl-对钙钛矿薄膜晶型和覆盖率的影响。通过对比SEM可以发现,当掺杂2% PbCl2时,钙钛矿膜更加致密,并且可以完整地覆盖TiO2层,如图 4所示。
 |
| a.MAPbI3沉积在TiO2介孔层上平面图;b.PbI2前驱体溶液中添加2% PbCl2的平面图;c.MAPbI3沉积在TiO2介孔层上断面图;d.PbI2前驱体溶液中添加2% PbCl2的断面图. 图 4 钙钛矿SEM分析 |
通过XRD衍射分析表明(220)/(310) 峰强度的比例会由于PbCl2的添加变强(如图 5所示),这解释了Cl-对MAPbI3晶粒生长的取向有一定的影响。
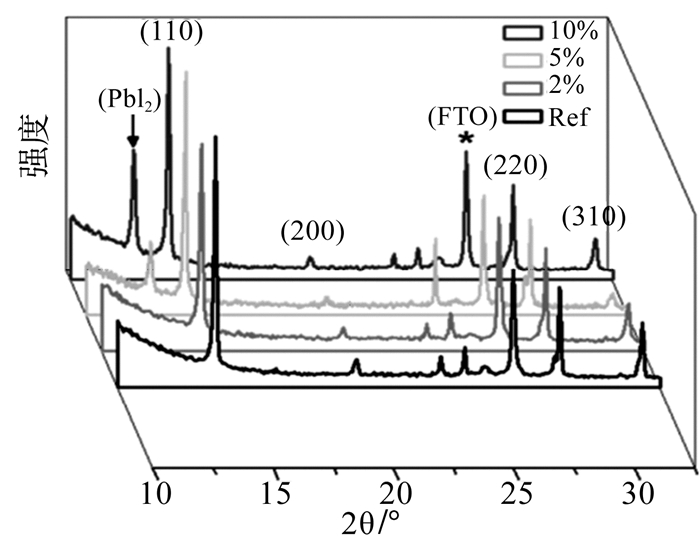 |
| 图 5 使用不同浓度PbCl2在TiO2上制备的MAPbI3的XRD衍射分析[50] |
ZHAO等人[51]将MACl添加到MAI和PbI2一步法溶液中,发现MACl可以延缓钙钛矿的结晶过程。MACl使钙钛矿在(110) 峰处强度增大,峰宽变窄,说明钙钛矿的结晶性更好。根据对添加MACl的钙钛矿薄膜不同加热时间下的XRD对比,猜测中间相MAI·(PbI2)x·MACl的形成延缓了钙钛矿结晶,但随加热时间延长,中间相最终消失。
基于上述研究结果,ZHAO等人[52]发现:在一步法PbI2和MABr前驱体溶液中加入一定量的MACl同样可以延缓结晶,有助于形成致密连续的薄膜。MACl的作用相当于粘结剂,形成PbI2-MABrx-MACl中间相,MACl在加热过程中升华,从而形成的MAPbI2Br能在基底致密层TiO2/FTO上达到全覆盖。对于Cl掺杂体系,先前的研究都只是集中在电池器件的性能测试上,而对Cl对器件性能影响的机理并不确定。YU等人[53]对一步法溶液(PbCl2与MAI的摩尔比为1:3) 制备钙钛矿薄膜时的Cl在结晶过程中的作用进行了探索,并提出MACl气体的挥发减缓了钙钛矿结晶。通过比较MAI/PbCl2和MAI/PbI2不同摩尔比的两种前驱体溶液,发现MA+富集对延缓结晶并在加热过程中促进晶体生长有重要作用,而Cl的作用是在相对较低的退火温度下去除多余的MA+。
MOSCONI等人[54]发现界面的Cl原子提高了MAPbI3-xClx(110) 晶面与TiO2的结合能,并且MAPbI3-xClx钙钛矿与TiO2的接触调整了界面电子结构。2013年,DOCAMPO等人[55]参照有机太阳能电池的结构组成,使用PEDOT为空穴传输层,PCBM和TiOx为电子传输层,制备光吸收层MAPbI(3-x)Clx,制备反型钙钛矿太阳能电池,器件的光电转化效率达到9.8%,其制备的柔性器件光电转化效率为6.3%。2014年,YOU等人[56]把这种反型结构应用于刚性和柔性器件,光电转化效率分别提高到10.53%和9.2%。
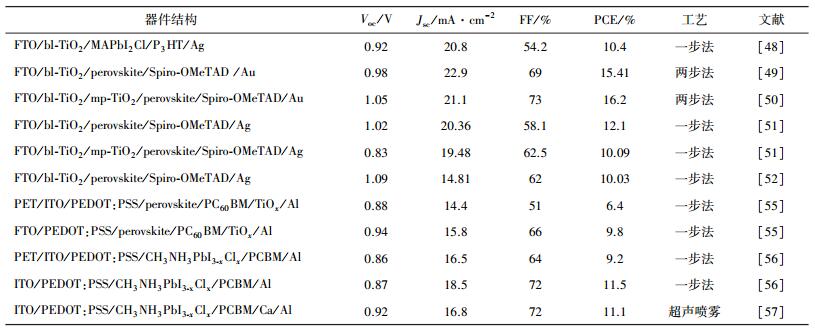
现今,钙钛矿层的成膜工艺广泛采用热分解方法。BARROWS等人[57]采用超声喷雾成膜工艺制备MAPbI(3-x)Clx薄膜,并应用于反型器件中, 获得了11.1%的光电转化效率。基于MAPbI(3-x)Clx为光吸收层,MATTEOCCI等人[58]制备出面积为16.8 cm2的太阳能电池模块,获得了5.1%光电转化效率。在空气中100 mW/cm2AM1.5光照335 h,器件效率仍有初始效率的60%,这为钙钛矿太阳能电池后期的商业化奠定了一定的基础。一步法制备钙钛矿薄膜时,Cl的引入可以提高成膜连续性并且提高电子—空穴扩散长度。MAPbI3-xClx可被看作是MAPbI3中的I部分被Cl替换,但是通过能谱仪(EDS)很难在薄膜中检测到Cl元素。更重要的是,Cl离子掺杂提高了钙钛矿薄膜的水氧稳定性、导电率和电荷扩散长度(约1μm)等[6, 33, 56, 59]。为了方便比较,我们在表 2列出了最近几年钙钛矿光吸收层材料的Cl掺杂的性能研究。
| 表 2 钙钛矿太阳能电池MAPbI3-xClx层氯掺杂性能的影响 |
 |
2.2 A位阳离子的掺杂、替换
1995年,CHOI等人[60]首次报道了基于甲脒基的锡卤钙钛矿HC(NH2)2SnI3。近年,随着钙钛矿太阳能电池的发展,材料的选择也多种多样。其中,甲脒基(FA+)取代甲氨基(MA+)所得到的一种新的钙钛矿材料FAPbI3在电池器件的应用中越发表现出卓越的性能[8, 32, 61]。PELLET等人[61]使用MAI与FAI混合的异丙醇溶液,在MAPbI3中掺杂入部分FA+,扩展吸收范围。实验证明MA0.6FA0.4PbI3的性能最好,其转化效率高达14.52%。LEE等人[62]通过离子交换反应在FAPbI3层上又沉积了一层MAPbI3。由于在长波长范围吸收的提高,使电流密度从19.57 mA cm-2提高到了20.22 mAcm-2。MAPbI3层改善了原有FAPbI3层的缺陷,从而提高了电压,最终器件效率达到16.01%。MEI等人[63]利用5-氨基戊酸(5-AVA)部分取代MA+获得(5-AVA)x(MA)1-xPbI3钙钛矿材料。用碳电极取代金电极和空穴传输层,器件的光电转化效率达到12.8%并表现出良好的器件稳定性。5-AVA中阳离子通过与I-的氢键作用影响钙钛矿的结晶,并且5-AVA中的阳离子可以作为钙钛矿结晶的模板,使得混合钙钛矿(5-AVA)x(MA)1-xPbI3层缺陷态减少,进而提高了激子寿命和电子传输效率。
MCMEEKIN等人[64]分别将Cs+和MA+,I-和Br-进行杂化,制备出一种混合型的钙钛矿材料[HC(NH2)2]0.83Cs0.17Pb(I0.6Br0.4)3, 带隙达到1.74 eV。CHOI等人[60]在MAPbI3中掺杂一定量的Cs,得到CsxMA(1-x)PbI3反型钙钛矿太阳能电池,并探究了Cs的掺杂量对器件的影响,获得了7.68%的光电转化效率。KULBAK等人[65]使用Cs完全代替MA+,将这种全无机的钙钛矿材料CsPbBr3用作光吸收层,获得6.2%的光电转化效率,并且表现出了较好的稳定性。YI等人[66]使用Cs部分掺杂入FAPbI3钙钛矿材料中,控制掺杂量为20%,初步获得了15.69%的转化效率;进一步对卤素原子进行部分Br替换,提高材料带隙,最终将Cs0.2FA0.8PbI2.84Br0.16作为光吸收层材料,获得了17.35%的光电转化效率。Cs的掺杂同样提高了电池器件的稳定性。
2.3 B位二价阳离子的掺杂、替换钙钛矿材料中金属离子通常是Pb2+,而铅元素为有毒重金属。Sn2+极易被氧化成Sn4+,形成自掺杂, 影响钙钛矿薄膜的稳定性,电池无法获得稳定性和高效率[67-69]。而Ge[70]和Bi[71]取代Pb后,由于溶解性和载流子复合等原因,光电转化效率极低。HAO等人[67]采用Sn代替Pb制备出CH3NH3SnI3-xBrx材料,通过改变I与Br的原子比,CH3NH3SnI3-xBrx的能隙可在1.30~2.15 eV内调控,器件效率达到了5.73%。同时,NOEL等人[72]进一步制备了全锡取代的钙钛矿材料CH3NH3SnI3,进而组装的超结构电池取得超过6%的电池效率。
3 总结与展望目前,钙钛矿太阳能电池认证效率已经达到了22.1%,其光电转化效率可与商业化晶硅电池相媲美。钙钛矿太阳能电池具有高效率、低成本、制备工艺简单等特点,展现出巨大的发展潜力。但是钙钛矿太阳能电池距离大规模商业化应用的目标还有较大距离。首要原因是现阶段具有较高光电转化效率的电池器件有效面积较小,这主要局限于大面积钙钛矿成膜质量。其次是钙钛矿太阳能电池的稳定性,这主要受其材料本质的影响。而且如何有效进行封装仍然亟待解决。另外,基于柔性基底的钙钛矿太阳能电池,由于其低温制备工艺以及易与塑料、织物布料等柔性基底相结合,成为可穿戴、便携式等柔性能源器件的不二选择,展现出巨大的魅力。通过掺杂工艺有效调节其能带,改善界面接触,从结构工程、材料工程出发,制备出的层叠太阳能电池,有望真正成为新一代光伏领域的主流产品。
| [1] |
GREEN M A. Third generation photovoltaics[M]. New York: Springer, 2006.
|
| [2] |
GREEN M A, HO-BAILLIE A, SNAITH H J. The emergence of perovskite solar cells[J]. Nature Photonics, 2014, 8(7): 506-514. DOI:10.1038/nphoton.2014.134 |
| [3] |
BISQUERT J. The swift surge of perovskite photovoltaics[J]. The Journal of Physical Chemistry Letters, 2013, 4(15): 2597-2598. DOI:10.1021/jz401435d |
| [4] |
STOUMPOS C C, MALLIAKAS C D, KANATZIDIS M G. Semiconducting tin and lead iodide perovskites with organic cations:phase transitions, high mobilities, and near-infrared photoluminescent properties[J]. Inorg Chem, 2013, 52(15): 9019-9038. DOI:10.1021/ic401215x |
| [5] |
STRANKS S D, EPERON G E, GRANCINI G, et al. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber[J]. Science, 2013, 342(6156): 341-344. DOI:10.1126/science.1243982 |
| [6] |
BALL J M, LEE M M, HEY A, et al. Low-temperature processed meso-superstructured to thin-film perovskite solar cells[J]. Energy & Environmental Science, 2013, 6(6): 1739-1743. |
| [7] |
NOH J H, IM S H, HEO J H, et al. Chemical management for colorful, efficient, and stable inorganic-organic hybrid nanostructured solar cells[J]. Nano Lett, 2013, 13(4): 1764-1769. DOI:10.1021/nl400349b |
| [8] |
BALL J M, LEE M M, HEY A, et al. Low-temperature processed meso-superstructured to thin-film perovskite solar cells[J]. Energy & Environmental Science, 2013, 6(6): 1739-1743. |
| [9] |
LIU M, JOHNSTON M B, SNAITH H J. Efficient planar heterojunction perovskite solar cells by vapour deposition[J]. Nature, 2013, 501(7467): 395-398. DOI:10.1038/nature12509 |
| [10] |
CHEN Q, ZHOU H, HONG Z, et al. Planar heterojunction perovskite solar cells via vapor-assisted solution process[J]. Journal of the American Chemical Society, 2014, 136(2): 622-625. DOI:10.1021/ja411509g |
| [11] |
BURSCHKA J, PELLET N, MOON S J, et al. Sequential deposition as a route to high-performance perovskite-sensitized solar cells[J]. Nature, 2013, 499(7458): 316-319. DOI:10.1038/nature12340 |
| [12] |
KOJIMA A, TESHIMA K, SHIRAI Y, et al. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells[J]. Journal of the American Chemical Society, 2009, 131(17): 6050-6051. DOI:10.1021/ja809598r |
| [13] |
BⅡ D, YI C, LUO J, et al. Polymer-templated nucleation and crystal growth of perovskite films for solar cells with efficiency greater than 21%[J]. Nature Energy, 2016, 1: 16142. DOI:10.1038/nenergy.2016.142 |
| [14] |
SEO J, PARK S, CHAN KIM Y, et al. Benefits of very thin PCBM and LiF layers for solution-processed p-i-n perovskite solar cells[J]. Energy & Environmental Science, 2014, 7(8): 2642-2646. |
| [15] |
REGAN B, GRATZEL M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films[J]. Nature, 1991, 353(6346): 737-740. DOI:10.1038/353737a0 |
| [16] |
WEI X, LIU J, CHUA Y Z, et al. Fabrication of O (dye)-terminated anatase TiO2 nanosheets for dye sensitized solar cells[J]. Energy & Environmental Science, 2011, 4(6): 2054-2057. |
| [17] |
WEI X, LIU J, LIU X-W. Ultrafine dice-like anatase TiO2 for highly efficient dye-sensitized solar cells[J]. Solar Energy Materials and Solar Cells, 2015, 134: 133-139. DOI:10.1016/j.solmat.2014.11.020 |
| [18] |
SUN S, SALIM T, MATHEWS N, et al. The origin of high efficiency in low-temperature solution-processable bilayer organometal halide hybrid solar cells[J]. Energy & Environmental Science, 2014, 7(1): 399-407. |
| [19] |
BAIKIE T, FANG Y, KADRO J M, et al. Synthesis and crystal chemistry of the hybrid perovskite (CH3NH3)PbI3 for solid-state sensitised solar cell applications[J]. Journal of Materials Chemistry A, 2013, 1(18): 5628-5641. DOI:10.1039/c3ta10518k |
| [20] |
EDRI E, KIRMAYER S, CAHEN D, et al. High open-circuit voltage solar cells based on organic-inorganic lead bromide perovskite[J]. J Phys Chem Lett, 2013, 4(6): 897-902. DOI:10.1021/jz400348q |
| [21] |
SINGH S P, NAGARJUNA P. Organometal halide perovskites as useful materials in sensitized solar cells[J]. Dalton Trans, 2014, 43(14): 5247-5251. DOI:10.1039/c3dt53503g |
| [22] |
LUAN M, SONG J, WEI X, et al. Controllable growth of bulk cubic-phase CH3NH3PbI3single crystal with exciting room-temperature stability[J]. CrystEngComm, 2016, 18(28): 5257-5261. DOI:10.1039/C6CE00375C |
| [23] |
栾梦雨, 刘晓倩, 陈方, 等. 有机-无机钙钛矿晶体生长调控研究进展[J]. 河南大学学报(自然科学版), 2016, 46(3): 276-285. |
| [24] |
CHUNG I, LEE B, HE J, et al. All-solid-state dye-sensitized solar cells with high efficiency[J]. Nature, 2012, 485(7399): 486-489. DOI:10.1038/nature11067 |
| [25] |
MENG L, YOU J, GUO T F, et al. Recent Advances in the inverted planar structure of perovskite solar cells[J]. Acc Chem Res, 2016, 49(1): 155-165. DOI:10.1021/acs.accounts.5b00404 |
| [26] |
KIM H S, LEE C R, IM J H, et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%[J]. Scientific Reports, 2012, 2: 591-598. DOI:10.1038/srep00591 |
| [27] |
LEIJTENS T, LAUBER B, EPERON G E, et al. The importance of perovskite pore filling in organometal mixed halide sensitized TiO2-based solar cells[J]. The Journal of Physical Chemistry Letters, 2014, 5(7): 1096-1102. DOI:10.1021/jz500209g |
| [28] |
BI D, BOSCHLOO G, SCHWARZMULLER S, et al. Efficient and stable CH3NH3PbI3-sensitized ZnO nanorod array solid-state solar cells[J]. Nanoscale, 2013, 5(23): 11686-11691. DOI:10.1039/c3nr01542d |
| [29] |
ZHONG D, CAI B, WANG X, et al. Synthesis of oriented TiO2 nanocones with fast charge transfer for perovskite solar cells[J]. Nano Energy, 2015, 11: 409-418. DOI:10.1016/j.nanoen.2014.11.014 |
| [30] |
TIAN H, XU B, CHEN H, et al. Solid-state perovskite-sensitized p-type mesoporous nickel oxide solar cells[J]. ChemSusChem, 2014, 7(8): 2150-2153. DOI:10.1002/cssc.201402032 |
| [31] |
WANG K C, JENG J Y, SHEN P S, et al. p-type Mesoscopic nickel oxide/organometallic perovskite heterojunction solar cells[J]. Scientific Reports, 2014, 4: 4756-4754. |
| [32] |
BI D, MOON S J, HAGGMAN L, et al. Using a two-step deposition technique to prepare perovskite (CH3NH3PbI3) for thin film solar cells based on ZrO2 and TiO2 mesostructures[J]. RSC Advances, 2013, 3(41): 18762-18766. DOI:10.1039/c3ra43228a |
| [33] |
LEE M M, TEUSCHER J, MIYASAKA T, et al. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites[J]. Science, 2012, 338(6107): 643-647. DOI:10.1126/science.1228604 |
| [34] |
HEO J H, IM S H, NOH J H, et al. Efficient inorganic-organic hybrid heterojunction solar cells containing perovskite compound and polymeric hole conductors[J]. Nature Photonics, 2013, 7(6): 486-491. DOI:10.1038/nphoton.2013.80 |
| [35] |
JENG J Y, CHIANG Y F, LEE M H, et al. CH3NH3PbI3 perovskite/fullerene planar-heterojunction hybrid solar cells[J]. Advanced Materials, 2013, 25(27): 3727-3732. DOI:10.1002/adma.v25.27 |
| [36] |
HUANG C, FU N, LIU F, et al. Highly efficient perovskite solar cells with precursor composition-dependent morphology[J]. Solar Energy Materials and Solar Cells, 2016, 145: 231-237. DOI:10.1016/j.solmat.2015.10.032 |
| [37] |
SANEHIRA E M, TREMOLET D E VILLERS B J, SCHULZ P, et al. Influence of electrode interfaces on the stability of perovskite solar cells:reduced degradation using MoOx/Al for hole collection[J]. ACS Energy Letters, 2016, 1(1): 38-45. DOI:10.1021/acsenergylett.6b00013 |
| [38] |
KULKARNI S A, BAIKIE T, BOIX P P, et al. Band-gap tuning of lead halide perovskites using a sequential deposition process[J]. Journal of Materials Chemistry A, 2014, 2(24): 9221-9225. DOI:10.1039/C4TA00435C |
| [39] |
EPERON G E, STRANKS S D, MENELAOU C, et al. Formamidinium lead trihalide:a broadly tunable perovskite for efficient planar heterojunction solar cells[J]. Energy & Environmental Science, 2014, 7(3): 982-988. |
| [40] |
KITAZAWA N, WATANABE Y, NAKAMURA Y. Optical properties of CH3NH3PbX3 (X=halogen) and their mixed-halide crystals[J]. Journal of Materials Science, 2002, 37(17): 3585-3587. DOI:10.1023/A:1016584519829 |
| [41] |
CAI B, XING Y, YANG Z, et al. High performance hybrid solar cells sensitized by organolead halide perovskites[J]. Energy & Environmental Science, 2013, 6(5): 1480-1485. |
| [42] |
JEON N J, NOH J H, YANG W S, et al. Compositional engineering of perovskite materials for high-performance solar cells[J]. Nature, 2015, 517(7535): 476-480. DOI:10.1038/nature14133 |
| [43] |
CAO K, CUI J, ZHANG H, et al. Efficient mesoscopic perovskite solar cells based on the CH3NH3PbI2Br light absorber[J]. Journal of Materials Chemistry A, 2015, 3(17): 9116-9122. DOI:10.1039/C5TA01129A |
| [44] |
BI C, YUAN Y, FANG Y, et al. Low-Temperature Fabrication of Efficient Wide-Bandgap Organolead Trihalide Perovskite Solar Cells[J]. Advanced Energy Materials, 2015, 5(6): 1616-1621. |
| [45] |
ZHU W, BAO C, LI F, et al. An efficient planar-heterojunction solar cell based on wide-bandgap CH3NH3PbI2.1Br0.9 perovskite film for tandem cell application[J]. Chemical Communications, 2016, 52(2): 304-307. DOI:10.1039/C5CC07673K |
| [46] |
JEON N J, NOH J H, KIM Y C, et al. Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells[J]. Nature Materials, 2014, 13(9): 897-903. DOI:10.1038/nmat4014 |
| [47] |
YIN M, XIE F, CHEN H, et al. Annealing-free perovskite films by instant crystallization for efficient solar cells[J]. Journal of Materials Chemistry A, 2016, 4(22): 8548-8553. DOI:10.1039/C6TA02490D |
| [48] |
CONINGS B, BAETEN L, DE DOBBELAERE C, et al. Perovskite-based hybrid solar cells exceeding 10% efficiency with high reproducibility using a thin film sandwich approach[J]. Advanced Materials, 2014, 26(13): 2041-2046. DOI:10.1002/adma.201304803 |
| [49] |
DOCAMPO P, HANUSCH F C, STRANKS S D, et al. Solution deposition-conversion for planar heterojunction mixed halide perovskite solar cells[J]. Advanced Energy Materials, 2014, 4(14): 355-361. |
| [50] |
DAR M I, ABDI-JALEBI M, ARORA N, et al. Growth engineering of CH3NH3PbI3structures for high-efficiency solar cells[J]. Advanced Energy Materials, 2016, 6(2): 1358-1366. |
| [51] |
ZHAO Y, ZHU K. CH3NH3Cl-assisted one-step solution growth of CH3NH3PbI3:structure, charge-carrier dynamics, and photovoltaic properties of perovskite solar cells[J]. The Journal of Physical Chemistry C, 2014, 118(18): 9412-9418. DOI:10.1021/jp502696w |
| [52] |
ZHAO Y, ZHU K. Efficient planar perovskite solar cells based on 1.8 eV band gap CH3NH3PbI2Br nanosheets via thermal decomposition[J]. Journal of the American Chemical Society, 2014, 136(35): 12241-12244. DOI:10.1021/ja5071398 |
| [53] |
YU H, WANG F, XIE F, et al. The Role of Chlorine in the Formation Process of "CH3NH3PbI3-xClx" Perovskite[J]. Advanced Functional Materials, 2014, 24(45): 7102-7108. |
| [54] |
MOSCONI E, RONCA E, ANGELIS F. First-principles investigation of the TiO2/organohalide perovskites interface:the role of interfacial chlorine[J]. The journal of physical chemistry letters, 2014, 5(15): 2619-2625. DOI:10.1021/jz501127k |
| [55] |
DOCAMPO P, BALL J M, DARWICH M, et al. Efficient organometal trihalide perovskite planar-heterojunction solar cells on flexible polymer substrates[J]. Nature Communications, 2013, 4(7): 657-678. |
| [56] |
YOU J, HONG Z, YANG Y M, et al. Low-temperature solution-processed perovskite solar cells with high efficiency and flexibility[J]. ACS Nano, 2014, 8(2): 1674-1680. DOI:10.1021/nn406020d |
| [57] |
BARROWS A T, PEARSON A J, KWAK C K, et al. Efficient planar heterojunction mixed-halide perovskite solar cells deposited via spray-deposition[J]. Energy & Environmental Science, 2014, 7(9): 2944-2950. |
| [58] |
MATTEOCCI F, RAZZA S, GIACOMO F, et al. Solid-state solar modules based on mesoscopic organometal halide perovskite:a route towards the up-scaling process[J]. Physical Chemistry Chemical Physics, 2014, 16(9): 3918-3923. DOI:10.1039/c3cp55313b |
| [59] |
COLELLA S, MOSCONI E, FEDELI P, et al. MAPbI3-xClx mixed halide perovskite for hybrid solar cells:the role of chloride as dopant on the transport and structural properties[J]. Chemistry of Materials, 2013, 25(22): 4613-4618. DOI:10.1021/cm402919x |
| [60] |
CHOI H, JEONG J, KIM H-B, et al. Cesium-doped methylammonium lead iodide perovskite light absorber for hybrid solar cells[J]. Nano Energy, 2014, 7: 80-85. DOI:10.1016/j.nanoen.2014.04.017 |
| [61] |
PELLET N, GAO P, GREGORI G, et al. Mixed-organic-cation perovskite photovoltaics for enhanced solar-light harvesting[J]. Angewandte Chemie International Edition, 2014, 53(12): 3151-3157. DOI:10.1002/anie.201309361 |
| [62] |
LEE J W, SEOL D J, CHO A N, et al. High-efficiency perovskite solar cells based on the black polymorph of HC(NH2)2 PbI3[J]. Advanced Materials, 2014, 26(29): 4991-4998. DOI:10.1002/adma.201401137 |
| [63] |
MEI A, LI X, LIU L, et al. A hole-conductor-free, fully printable mesoscopic perovskite solar cell with high stability[J]. Science, 2014, 345(6194): 295-298. DOI:10.1126/science.1254763 |
| [64] |
MCMEEKIN D P, SADOUGHI G, REHMAN W, et al. A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells[J]. Science, 2016, 351(6269): 151-155. DOI:10.1126/science.aad5845 |
| [65] |
KULBAK M, GUPTA S, KEDEM N, et al. Cesium enhances long-term stability of lead bromide perovskite-based solar cells[J]. The Journal of Physical Chemistry Letters, 2015, 7(1): 167-172. |
| [66] |
YI C, LUO J, MELONI S, et al. Entropic stabilization of mixed A-cation ABX3 metal halide perovskites for high performance perovskite solar cells[J]. Energy & Environmental Science, 2016, 9(2): 656-662. |
| [67] |
HAO F, STOUMPOS C C, CAO D H, et al. Lead-free solid-state organic-inorganic halide perovskite solar cells[J]. Nature Photonics, 2014, 8(6): 489-494. DOI:10.1038/nphoton.2014.82 |
| [68] |
HAO F, STOUMPOS C C, CHANG R P, et al. Anomalous band gap behavior in mixed Sn and Pb perovskites enables broadening of absorption spectrum in solar cells[J]. Journal of the American Chemical Society, 2014, 136(22): 8094-8099. DOI:10.1021/ja5033259 |
| [69] |
ZUO F, WILLIAMS S T, LIANG P W, et al. Binary-metal perovskites toward high-performance planar-heterojunction hybrid solar cells[J]. Advanced Materials, 2014, 26(37): 6454-6460. DOI:10.1002/adma.201401641 |
| [70] |
KRISHNAMOORTHY T, DING H, YAN C, et al. Lead-free germanium iodide perovskite materials for photovoltaic applications[J]. Journal of Materials Chemistry A, 2015, 3(47): 23829-23832. DOI:10.1039/C5TA05741H |
| [71] |
PARK B W, PHILIPPE B, ZHANG X, et al. Bismuth based hybrid perovskites A3Bi2I9 (A:methylammonium or cesium) for solar cell application[J]. Advanced Materials, 2015, 27(43): 6806-6813. DOI:10.1002/adma.201501978 |
| [72] |
NOEL N K, STRANKS S D, ABATE A, et al. Lead-free organic-inorganic tin halide perovskites for photovoltaic applications[J]. Energy & Environmental Science, 2014, 7(9): 3061-3068. |
 2016, Vol. 30
2016, Vol. 30


