聚N-异丙基丙烯酰胺(PNIPAM)是典型的温敏刺激响应性聚合物之一,在低临界溶解温度(LCST)附近呈现可逆的相结构和亲水/亲油性转变[1~3]。将PNIPAM键合到聚氯乙烯(PVC)分子链上,不仅可提高PVC的亲水、生物相容和抗凝血性能,而且可赋予材料温敏性,拓展PVC在医用器械、温敏型胶束和水凝胶中的应用。PNIPAM与PVC键合的主要方式有接枝和嵌段共聚。在接枝共聚方面,Yoshioka等[4]通过取代反应先将N, N-二乙基二硫代氨基甲酸钠接枝到PVC分子链上,再通过紫外光引发N-异丙基丙烯酰胺(NIPAM)聚合,得到PVC-g-PNIPAM共聚物,用该共聚物涂布的聚对苯二甲酸乙二醇酯薄膜的水接触角在PNIPAM的LCST附近发生突变。Lee等[5]以脱除少量氯化氢的PVC为接枝基体,通过自由基接枝共聚制备了PVC-g-PNIPAM共聚物,并比较了PVC/PNIPAM共混物和PVC-g-PNIPAM共聚物中PVC和PNIPAM的相容性、接触角和力学性能。Arenas等[6]采用60Co-γ射线引发PVC管表面的NIPAM接枝共聚,改性PVC管具有温敏性水溶胀特性。本课题组[7]采用单电子转移活性自由基聚合实现了NIPAM在PVC上的高效接枝,得到PNIPAM接枝密度和接枝链长度对共聚物胶束尺寸和LCST的影响规律,并发现接枝共聚物胶束可在极低浓度下发生温敏性聚集。含PNIPAM链段的嵌段共聚物多采用活性自由基聚合(LRP)方法制备,尽管由PNIPAM和非PVC疏水链段(如聚苯乙烯、聚丙烯酸正丁酯或聚丁二烯等)构成的嵌段共聚物的合成和自组装已有报道[8~13],但有关PVC-PNIPAM嵌段共聚物的合成与特性研究未见报道。
单电子转移-蜕化链转移活性自由基聚合(SET-DT LRP)是Percec等提出的以碘仿等卤代烷烃为引发剂、连二亚硫酸钠或铜/配体为催化剂的LRP方法,已成功用于氯乙烯、(甲基)丙烯酸酯类等单体聚合[14]。通过SET-DT LRP方法先合成端基为碘的活性PVC或聚丙烯酸酯,再进一步引发丙烯酸酯或氯乙烯聚合可制得各种聚氯乙烯-聚丙烯酸酯嵌段共聚物[15~21]。本课题组通过以碘仿为引发剂、Na2S2O4/NaHCO3为催化体系的SET-DT LRP方法合成了聚氯乙烯-聚丙烯酸嵌段共聚物,并与PVC共混制备了具有pH响应性的PVC超滤膜[22]。本文采用SET-DT LRP方法合成了不同组成的PNIPAM-b-PVC-b-PNIPAM三嵌段共聚物,并研究了共聚物组成对其水相自组装行为(临界胶束浓度、胶束结构及LCST等)的影响。
2 实验(材料和方法) 2.1 试剂N, N-二甲基甲酰胺(DMF)、Na2S2O4、NaHCO3、乙醚等为分析纯,碘仿(CHI3)为化学纯,国药集团化学试剂有限公司;VC,聚合级,杭州电化集团有限公司;NIPAM(纯度98%)、芘(纯度98%),上海百灵威科技有限公司;分散剂羟丙基甲基纤维素(HPMC)、甲基纤维素(MC),Dow化学公司。
2.2 I-PVC-I的合成在5 L不锈钢耐压反应釜中加入20.0 g CHI3、1.5 L去离子水、1.0 g HPMC及0.43 g MC,密封反应釜,以高纯氮置换釜内氧气5次,加入500 g VC;在700 r·min-1转速下搅拌并控温至(35±0.2)℃;压入100 mL溶有Na2S2O4(27.8 g)和NaHCO3(9.0 g)的去离子水溶液,开始反应;约8 h后结束聚合反应,过滤、洗涤,室温干燥得到I-PVC-I。
2.3 PNIPAM-b-PVC-b-PNIPAM共聚物的合成典型过程如下:将2.0 g I-PVC-I、7.24 g NIPAM(0.064 mol)加入到含有80 mL DMF的三口玻璃夹套釜中,用50℃水浴加热并搅拌,排气30 min后加入0.056 g (0.32 mmol)Na2S2O4及0.054 g(0.64 mmol)NaHCO3,反应3 h,以体积10倍于反应液的乙醚作沉淀剂沉淀产物,并以去离子水洗涤、45℃干燥至恒重。
2.4 PNIPAM-b-PVC-b-PNIPAM共聚物胶束的制备以5 mL DMF溶解20 mg共聚物,逐滴滴加至10 mL去离子水中,透析48 h,每3 h换水一次,最终将共聚物胶束溶液定容至20 mL。
2.5 表征共聚物化学结构采用500 MHz Bruker DRX500核磁共振(NMR)表征,使用THF-d8作溶剂。聚合物平均分子量及分子量分布采用Waters1525/2414型凝胶渗透色谱(GPC)测定,以浓度为0.05 mol·L-1的LiBr的DMF溶液为洗脱液,聚合物浓度为0.5%(wt),以窄分子量分布聚甲基丙烯酸甲酯为标样。
采用Shimadzu RF-6000 Spectro荧光光谱仪测定PNIPAM-b-PVC-b-PNIPAM共聚物的临界胶束浓度(CMC),芘为荧光探针分子,芘溶液浓度为5.0×10-7mol·L-1。分别配制浓度为0.0001、0.0002、0.0004、0.0008、0.001、0.002、0.004、0.01、0.02、0.04、0.1、0.5、1.0 g·L-1的共聚物胶束溶液用于分析。共聚物胶束溶液的吸光度采用紫外-可见分光光度计(岛津UV-1800)测定,波长500 nm,升温速率为0.5℃·min-1。共聚物胶束流体力学直径采用Malvern Zetasizer Nano ZS90动态光散射(DLS)测定,散射角90°,温度25℃。将胶束溶液滴于镀有碳膜的铜网,室温下烘干,采用JEM-1230透射电子显微镜(TEM)观察胶束形貌,加速电压80 kV。
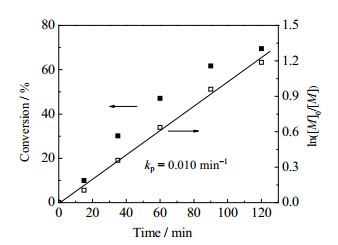
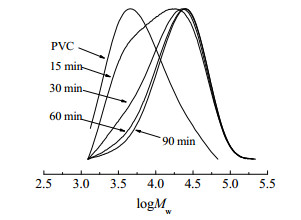
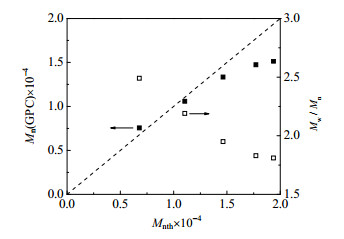
3 结果与讨论 3.1 PNIPAM-b-PVC-b-PNIPAM共聚物的合成采用SET-DT LRP方法聚合得到数均分子量(Mn)为4670(平均聚合度75)、分子量分布指数(Mw/Mn)为2.31,和Mn为10680(平均聚合度171)、Mw/Mn为2.48的两种I-PVC-I,再以I-PVC-I为大分子引发剂进行NIPAM聚合制备两亲性嵌段共聚物。为了验证SET-DT聚合的活性,考察了I-PVC-I引发的NIPAM聚合的动力学。图 1为NIPAM转化率与ln([M]0/[M])随聚合时间的变化曲线,其中[M]0与[M]分别是反应时间t=0与t=t时的NIPAM摩尔浓度。可见,ln([M]0/[M])随着聚合时间线性增加,符合活性自由基聚合的典型动力学。图 2为I-PVC-I和不同NIPAM聚合时间得到的PNIPAM-b-PVC-b-PNIPAM共聚物的分子量分布曲线。可见,共聚物分子量呈单峰分布,随着聚合时间增加,分子量分布曲线逐渐向大分子量方向移动。图 3为PNIPAM-b-PVC-b-PNIPAM共聚物理论Mn与实测Mn的比较和Mw/Mn值的变化。由于反应体系的非均相特性,在低转化率(低分子量)阶段,理论和实测Mn接近,而到高转化率(高分子量)阶段,实测Mn略低于理论Mn;随着分子量增加,共聚物的Mw/Mn值逐渐减小,这也表明了NIPAM嵌段共聚的活性。
|
图 1 NIPAM聚合转化率和ln([M]0/[M])随聚合时间的变化 Fig.1 Variation of conversion and ln([M]0/[M]) as a function of time in NIPAM polymerization (I-PVC-I Mn=4670, [VC]:[NIPAM]=1: 2.5, [NIPAM]:[Na2S2O4]=250:1) |
|
图 2 I-PVC-I和不同聚合时间得到的PNIPAM-b-PVC-b-PNIPAM共聚物的GPC谱线 Fig.2 GPC spectra of I-PVC-I and PNIPAM-b-PVC-b-PNIPAM copolymers obtained at different polymerization times (I-PVC-I Mn=4670, [VC]:[NIPAM]=1:2.5, [NIPAM]:[Na2S2O4]=250:1) |
|
图 3 PNIPAM-b-PVC-b-PNIPAM共聚物理论Mn和实测Mn的比较和Mw/Mn值变化 Fig.3 Comparison on theoretical and GPC determined Mn and variation of Mw/Mn of PNIPAM-b-PVC-b-PNIPAM copolymer (I-PVC-I Mn=4670, [VC]:[NIPAM]=1:2.5, [NIPAM]:[Na2S2O4]=250:1) |
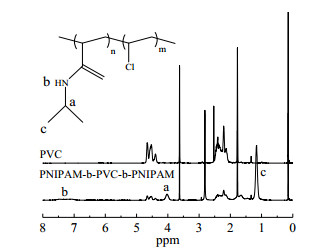
图 4为PNIPAM-b-PVC-b-PNIPAM共聚物与I-PVC-I的1H-NMR谱图。由图可见,大约在1.1(c)、4.0(a)、7.0~8.0(b)及4.5 ppm附近的位移峰,分别归属异丙基的甲基氢、次甲基氢、PNIPAM嵌段的酰胺基氢和PVC链上氯代亚甲基氢(-CHCl)。在I-PVC-I的1H-NMR谱δ=6.0ppm附近属于-CHClI基团的峰,在嵌段共聚物的1H-NMR谱中消失,表明了PNIPAM链段成功嵌段在I-PVC-I两端。
|
图 4 活性PVC和PNIPAM-b-PVC-b-PNIPAM共聚物的1H-NMR谱线 Fig.4 1H-NMR spectra of I-PVC-I and PNIPAM-b-PVC-b-PNIPAM copolymers |
PNIPAM-b-PVC-b-PNIPAM共聚物具有两亲特性,有可能在水中自组装形成以疏水PVC链段为核、亲水PNIPAM链段为壳的胶束。合成的PNIPAM-b-PVC-b-PNIPAM共聚物不溶于水,故采用溶剂交换法制备共聚物胶束。在300~380 nm波长范围内,不同浓度PNIPAM-b-PVC-b-PNIPAM共聚物胶束溶液的芘荧光激发光谱、激发光谱强度比I339/I334与胶束浓度对数(logC)的关系如图 5所示。可见,PNIPAM-b-PVC-b-PNIPAM共聚物胶束表现出从334到339 nm的红移现象,说明探针分子环境由亲水逐渐转变为疏水,表明了胶束的形成。
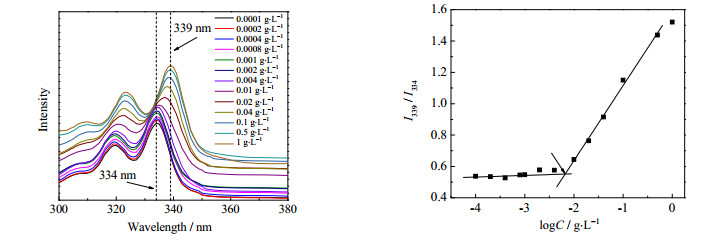
|
图 5 不同浓度PNIPAM42-b-PVC171-b-PNIPAM42共聚物胶束溶液的芘荧光激发光谱(25℃)和荧光激发光谱强度比I339/I334与log C关系 Fig.5 Fluorescence spectroscopy and intensity ratio (I338/I333)-log C relation for PNIPAM42-b-PVC171-b-PNIPAM42 copolymer micelle solutions containing pyrene |
I339/I334强度比急剧上升的浓度点对应着两亲共聚物溶液的CMC,当嵌段共聚物浓度小于CMC时,溶液中尚未形成胶束,所有芘分子都处于亲水环境中,此时I339/I334几乎不变。当嵌段共聚物浓度大于CMC,溶液中开始形成胶束,一定量的芘分子转移到胶束内部的疏水环境,334 nm处的激发峰开始发生偏移,I339/I334逐渐增大。拟合得到PNIPAM14-b-PVC171-b-PNIPAM14、PNIPAM38-b-PVC171-b-PNIPAM38与PNIPAM42-b-PVC171-b-PNIPAM42共聚物的CMC依次为5.6、6.3、6.6 mg·L-1。CMC依赖于共聚物的亲水/疏水性,随着亲水性PNIPAM链段含量的增加,CMC逐渐增大。三种共聚物的CMC都很低,因为三者的疏水性都很强,共聚物在水中的溶解度很低。
3.3 PNIPAM-b-PVC-b-PNIPAM共聚物胶束结构采用DLS和TEM分别分析了共聚物胶束的粒径分布和形貌,结果如图 6、7所示,体均粒径和粒径分布指数如表 1所示。由图 6和表 1可见,共聚物胶束的尺寸在75~134 nm,并随着亲水链段含量或亲水/疏水链段比的增加而逐渐降低。虽然共聚物分子链长增加,但亲水性逐渐增加,胶束聚集数逐渐降低,形成一个胶束所需的嵌段共聚物分子数降低。
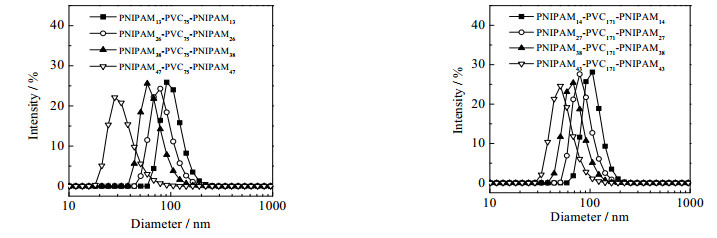
|
图 6 不同组成PNIPAM-b-PVC-b-PNIPAM共聚物胶束粒径分布 Fig.6 Micelle size distributions of PNIPAM-b-PVC-b-PNIPAM copolymers with different compositions (micelle concentration 0.1%(wt), 25℃) |
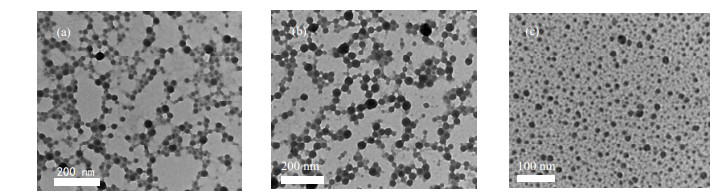
|
图 7 PNIPAM-b-PVC-b-PNIPAM共聚物胶束的TEM照片(0.1%(wt)) Fig.7 TEM micrographs of PNIPAM-b-PVC-b-PNIPAM copolymer micelles (0.1%(wt)) (a) PNIPAM14-b-PVC171-b-PNIPAM14 (b) PNIPAM13-b-PVC75-b-PNIPAM13 (c) PNIPAM43-b-PVC171-b-PNIPAM43 |
|
|
表 1 不同组成PNIPAM-b-PVC-b-PNIPAM共聚物胶束尺寸及分布指数(胶束浓度0.1%(wt)) Table 1 Volume average size and size distribution indexes of PNIPAM-b-PVC-b-PNIPAM copolymer micelles |
由图 7可见,PNIPAM-b-PVC-b-PNIPAM共聚物胶束呈球形,PNIPAM形成胶束的亲水性外壳,PVC形成胶束的疏水性内核。TEM照片所显示的胶束尺寸要小于DLS所测的胶束尺寸,因为干燥过程中胶束失水,在水溶液中舒展的亲水链段收缩,所以粒径变小。(a)图PNIPAM14-b-PVC171-b-PNIPAM14与(b)图PNIPAM13-b-PVC75-b-PNIPAM13胶束尺寸近似相等,(c)图PNIPAM43-b-PVC171-b-PNIPAM43胶束尺寸要小于(a)、(b),这与DLS所测结果相符。
3.4 PNIPAM-b-PVC-b-PNIPAM共聚物的低临界溶解温度因为PNIPAM的温敏性,PNIPAM-b- PVC-b-PNIPAM共聚物亦表现出LCST行为,随着温度的变化胶束会发生相转变,导致胶束溶液吸光度的变化,典型结果如图 8所示。
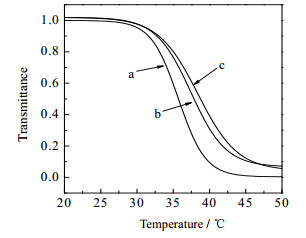
|
图 8 PNIPAM-b-PVC-b-PNIPAM共聚物溶液透光率随温度的变化(0.1%(wt))
Fig.8 Profiles of PNIPAM-b-PVC-b-PNIPAM copolymer solution transmittance as a function of temperature (0.1%(wt))
a. PNIPAM14-b-PVC171-b-PNIPAM14
b. PNIPAM27-b-PVC171-b-PNIPAM27 c. PNIPAM38-b-PVC171-b-PNIPAM38 |
当温度较低时,共聚物胶束溶液呈透明状,随着温度的升高,溶液变得浑浊,透光率逐渐下降。这是因为升温过程中PNIPAM的疏水性逐渐增加,胶束变得不稳定而聚集,同时胶束的溶剂化作用减弱,疏水相与水溶液环境之间的微相分离更加显著。LSCT受共聚物亲水/疏水性的影响,随着亲水性的增加,胶束的LCST逐渐增加,PNIPAM14-b- PVC171-b-PNIPAM14、PNIPAM27-b-PVC171-b-PNIPAM27和PNIPAM38-b-PVC171-b-PNIPAM38共聚物的LCST依次为35.6、37.2和38.3℃。
4 结论采用SET-DT LRP成功合成了不同组成的PNIPAM-b-PVC-b-PNIPAM两亲嵌段共聚物,并验证了I-PVC-I引发的NIPAM聚合的活性特征。PNIPAM-b-PVC-b-PNIPAM共聚物在水中能自组装形成以PVC为核、PNIPAM为壳的球形胶束。随着共聚物疏水PVC链段含量的增加,临界胶束浓度逐渐降低,胶束尺寸逐渐增大。因为PNIPAM的温敏性,PNIPAM-b-PVC-b-PNIPAM共聚物表现出LCST行为,随着共聚物亲水链段含量的增加,LCST逐渐增加。
| [1] | Hoffman A S, Stayton P S. Conjugates of stimuli-responsive polymers and proteins[J]. Progress in Polymer Science , 2007, 32(8-9): 922-932. DOI:10.1016/j.progpolymsci.2007.05.005. |
| [2] | Tian H Y, Tang Z H, Zhuang X L, et al. Biodegradable synthetic polymers:Preparation, functionalization and biomedical application[J]. Progress in Polymer Science , 2012, 37(2): 237-280. DOI:10.1016/j.progpolymsci.2011.06.004. |
| [3] | Roy D, Cambre J N, Sumerlin B S. Future perspectives and recent advances in stimuli-responsive materials[J]. Progress in Polymer Science , 2010, 35(1-2): 278-301. DOI:10.1016/j.progpolymsci.2009.10.008. |
| [4] | Yoshioka H, Mikami M, Mori Y, et al. Preparation of thermoresponisve surfaces using polyvinychloride-graft-poly (N-isopropylacrylamide)[J]. Polymer for Advanced Technologies , 1993, 4(1): 519-521. |
| [5] | Lee W F, Tu Y M. Graft copolymerization of N-isopropylacrylamide onto poly(vinyl chloride)[J]. Journal of Applied Polymer Science , 1999, 74(5): 1234-1241. DOI:10.1002/(ISSN)1097-4628. |
| [6] | Arenas E, Bucio E, Burillo G, et al. Radiation grafting of N-isopropylacrylamide onto poly(vinyl chloride) tubes by gramma irradiation[J]. Polymer Bulletin , 2007, 58(2): 401-409. DOI:10.1007/s00289-006-0672-6. |
| [7] | Liu K, Pan P, Bao Y. Synthesis, micellization, and thermally-induced macroscopic micelle aggregation of poly(vinyl chloride)-g-poly(N-isopropylacrylamide) amphiphilic copolymer[J]. RSC Advance , 2015, 5(115): 94582-94590. DOI:10.1039/C5RA16726D. |
| [8] | Cetintas M, Kamperman M. Self-assembly of PS-b-PNIPAM-b-PS block copolymer thin films via selective solvent annealing[J]. Polymer , 2016, 107(1): 387-397. |
| [9] | PU Xin-ming(蒲新明), JU Zhen-hua(琚振华), CHEN Qi-jing(陈起静), et al. Synthesis of amphiphilic highly branched block copolymers based on mechanism transformation from anionic polymerization Into RAFT-based polymerization(基于负离子-RAFT机理转换合成两亲性高度支化嵌段共聚物)[J]. Acta Polymerica Sinica(高分子学报) , 2017(2): 283-293. |
| [10] | Papagiannopoulos A, Meristoudi A, Pispas S, et al. Thermoresponsive behavior of micellar aggregates from end-functionalized PnBA-b-PNIPAM-COOH block copolymers and their complexes with lysozyme[J]. Soft Matter , 2016, 12(31): 6547-6556. DOI:10.1039/C6SM00976J. |
| [11] | Luo Y L, Yang X L, Xu F, et al. Thermosensitive PNIPAM-b-HTPB block copolymer micelles:molecular architectures and camptothecin drug release[J]. Colloids and Surfaces B:Biointerfaces , 2014, 114(1): 150-157. |
| [12] | Graisuwan W, Zhao H, Kiatkamjornwong S, et al. Formation of thermo-sensitive and cross-linkable micelles by self-assembly of poly(pentafluorophenyl acrylate)-containing block copolymer[J]. Journal of Polymer Science, Part A:Polymer Chemistry , 2015, 53(9): 1103-1113. DOI:10.1002/pola.v53.9. |
| [13] | Adelsberger J, Grillo I, Kulkarni A, et al. Kinetics of aggregation in micellar solutions of thermoresponsive triblock copolymers-influence of concentration, start and target temperatures[J]. Soft Matter , 2012, 9(5): 1685-1699. |
| [14] | Rosen B M, Percec V. Single-electron transfer and single-electron transfer degenerative chain transfer living radical polymerization[J]. Chemical Reviews , 2009, 109(11): 5069-5119. DOI:10.1021/cr900024j. |
| [15] | Percec V, Guliashvili T, Popov A V, et al. Synthesis of poly(methyl methacrylate)-b-poly(vinyl chloride)-b-poly(methyl methacrylate) block copolymers by CuCl/2, 2'-bipyridine-catalyzed living radical block copolymerization initiated from alpha, omega-di(iodo) poly(vinyl chloride) prepared by single-electron-transfer/degenerative-chain-transfer mediated living radical polymerization[J]. Journal of Polymer Science Part A-Polymer Chemistry , 2005, 43(7): 1478-1486. DOI:10.1002/(ISSN)1099-0518. |
| [16] | Percec V, Guliashvili T, Popov A V, et al. Ultrafast synthesis of poly(methyl methacrylate)-b-poly(vinyl chloride)-b-poly(methyl methacrylate) block copolymers by the Cu(0)/tris(2-dimethylaminoethyl)amine-catalyzed living radical block copolymerization of methyl methacrylate initiated with ɑ, ω-di(iodo)poly(vinyl chloride) in the presence of dimethyl sulfoxide at 25 degrees C[J]. Journal of Polymer Science Part A-Polymer Chemistry , 2005, 43(8): 1660-1669. DOI:10.1002/(ISSN)1099-0518. |
| [17] | Percec V, Popov A V, Ramirez-Castillo E, et al. Synthesis of poly(vinyl chloride)-b-poly(2-ethylhexyl acrylate)-b-poly(vinyl chloride) by the competitive single-electron-transfer/degenerative-chain-transfer mediated living radical polymerization of vinyl chloride initiated from ɑ, ω-di(iodo)poly(2-ethylhexyl acrylate) and catalyzed with sodium dithionite in water[J]. Journal of Polymer Science Part A-Polymer Chemistry , 2005, 43(11): 2276-2280. DOI:10.1002/(ISSN)1099-0518. |
| [18] | Coelho J F J, Silva A, Popov A V, et al. Synthesis of poly(vinyl chloride)-b-poly(n-butyl acrylate)-b-poly(vinyl chloride) by the competitive single-electron-transfer/degenerative-chain-transfer-mediated living radical polymerization in water[J]. Journal of Polymer Science Part A-Polymer Chemistry , 2006, 44(9): 3001-3008. DOI:10.1002/(ISSN)1099-0518. |
| [19] | Percec V, Sienkowska M J. Synthesis of the four-arm star-block copolymer PVC-b-PBA-CH(CH3)-CO-O-CH2(4)C by SET-DTLRP initiated from a tetrafunctional initiator[J]. Journal of Polymer Science Part A-Polymer Chemistry , 2009, 47(2): 628-634. DOI:10.1002/pola.v47:2. |
| [20] | Rocha N, Gamelas J A F, Goncalves P M, et al. Influence of physical-chemical interactions on the thermal stability and surface properties of poly(vinyl chloride)-b-poly(hydroxypropyl acrylate)-b-poly(vinyl chloride) block copolymers[J]. European Polymer Journal , 2009, 45(12): 3389-3398. DOI:10.1016/j.eurpolymj.2009.09.020. |
| [21] | Rocha N, Coelho J F J, Gois J R, et al. Poly(vinyl chloride)-b-poly(hydroxypropyl acrylate)-b-poly(vinyl chloride):understanding the synthesis of an amphiphilic PVC block copolymer on a pilot scale[J]. Journal of Vinyl & Additive Technology , 2013, 19(2): 94-104. |
| [22] | YIN Xun-di(尹逊迪), HUANG Zhi-hui(黄志辉), BAO Yong-zhong(包永忠). pH-Responsive poly(vinyl chloride) ultrafiltration membranes modified by poly(vinyl chloride)-poly(acrylic acid) block copolymers(聚氯乙烯-聚丙烯酸嵌段共聚物改性的pH响应性聚氯乙烯超滤膜)[J]. Journal of Chemical Engineering of Chinese Universities(高校化学工程学报) , 2017, 31(4): 938-944. |




