2. 重庆大学 山地城镇建设与新技术教育部重点实验室,重庆 400045
2. Key Laboratory of New Technology for Construction of Cities in Mountain Area, Ministry of Education, Chongqing University, Chongqing 400045, China
随着现代生活和工作方式的去群体化,人们处于室内环境的时间占比日益增加,特别是城市人群每天约有80 % 以上的时间在室内完成各项活动[1-3]。因此,室内空气品质对人体健康水平具有决定性影响。研究表明,当前室内甲醛和苯系化合物等常见气态污染物的浓度超标严重(最高达标准限值的10余倍)[4-6],污染持续周期可达数月乃至数年[7-9]。这不仅会严重损害人体心脑血管、呼吸以及免疫等功能系统,引发白血病、肺结核以及肝肾衰竭等恶性疾病[10-14],而且容易扩散至室外大气环境,造成区域性污染物浓度超标,影响周围空气、水域和植被等[15-17]。因此,亟需控制并降低室内气态污染物浓度。
在已有的室内空气净化方法中,通风换气是最常采用的方法。相较于其他方法,通风换气具有净化速率高[18-20]、作用范围广[21-23]等优点。然而,通风换气方法不完全适用于连续性污染场景[24]、室外干扰较大场景[25-26]等。因此,需要在实现室内空气净化的同时,综合考虑净化时效/范围等多种因素。
相较于通风换气方法,基于吸附净化的被动式捕集方法具有特殊优势并越来越受到重视:不仅可以长效净化[27-29],而且可以完成污染物吸附固定[30]。此外,吸附净化方法可以实现特定场景下室内气态污染物高效循环捕集[31]。因此,研究并开发基于吸附净化的被动式吸附捕集系统对于减少室内空气污染、提升室内空气品质具有重要意义。
吸附净化方法普遍具有捕集范围较广泛、吸附方式多元以及捕集性能差异较大等特点[32-34]。无论是制备更高效的吸附剂,还是开发更先进的吸附捕集技术,首先均需要在微/介观层面厘清相应的吸附机理并在宏观层面建立对应的技术逻辑。目前,室内气态污染物的吸附捕集研究以实验探究不同吸附剂的吸附性能为主,不仅缺乏从机理层面设计吸附剂/调控吸附过程的本征先驱条件,而且难以对应或应用于后续的室内吸附净化技术体系。针对这一状况,本文综述了常见室内气态污染物(以《室内空气质量标准》(GB/T 18883—2022)为准[35],甲醛(HCHO)≤8×10-5 g·m-3、苯(C6H6)≤3×10-5 g·m-3、甲苯(C6H5CH3)≤2×10-4 g·m-3、二甲苯(CH3C6H4CH3)≤2×10-4 g·m-3、总挥发性有机化合物(TVOC)≤6×10-4 g·m-3)的微观吸附机理,着重讨论了不同机理的特点和适用情况,并归纳总结了基于上述吸附机理的吸附技术及其优缺点,为后续气态污染物高效净化提供理论依据。
2 室内气态污染物吸附机理 2.1 物理/化学吸附物理/化学吸附是指吸附质(即甲醛、苯等气态污染物)与吸附剂通过分子间相互作用或化合反应等单向方式相结合(图 1)[36-38]。其中,分子间相互作用通常以范德华力和氢键的形式表现:范德华力主要取决于吸附系统的综合极性(本征偶极矩与瞬时偶极矩的矢量和),而氢键与结合位点的电负性相关[39]。由于分子间相互作用的强度一般较低,其通常属于物理吸附(以吸附能为“0~-96 500.00 J·mol-1”记作物理吸附[40]);对于化合反应方式,吸附质通过化学成键方式与吸附剂结合,结合方式取决于成键位置与类型。因成键强度差异较大,化合反应方式既可能属于物理吸附,又可能属于化学吸附(以吸附能为“-96 500.00~-∞ J·mol-1”记作化学吸附[40])。
与燃煤电厂等场景的工业烟气吸附净化不同,室内气态污染物吸附净化需要着重考虑以下2个要点:1)室内气态污染物浓度/含量极低。室内常见的总挥发性有机化合物质量浓度一般约为10-6 g·m-3量级[41],远低于工业烟气浓度[42];2)室内气态污染物的化学活性范围窄。相较于工业烟气中二氧化碳(CO2)/二氧化硫(SO2)/氮氧化物(NOx)等可通过气固、气液等特定化学反应进行捕集[43],甲醛/苯等室内污染物暂无专用的工业特效吸附剂。
基于上述物理/化学吸附原理和室内气态污染物的特点,目前常见的吸附机理可分为无差别共吸附和选择性吸附。
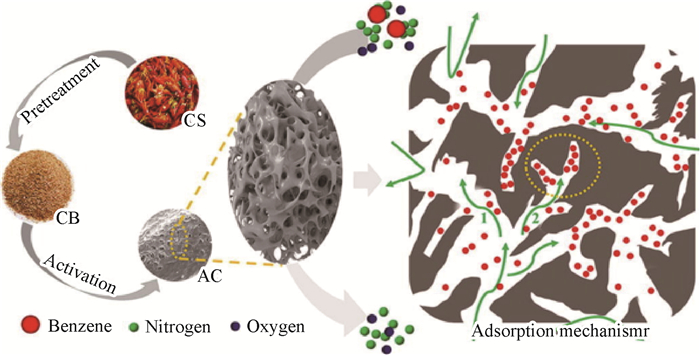
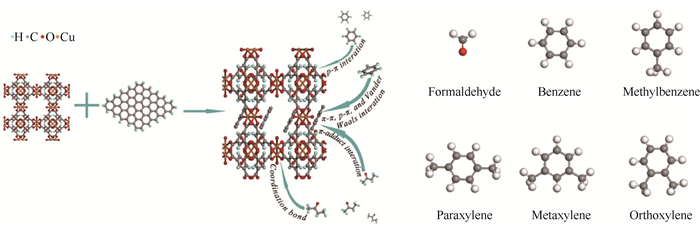
2.1.1 无差别共吸附方式无差别共吸附方法采用活性炭、金属有机框架等具有较大比表面/孔径的非选择极性材料作为吸附剂,通过调控吸附剂表面使之具有多个极性位点。当吸附质靠近时,这些极性位点会激发吸附质产生瞬时偶极矩,进而通过分子间相互作用吸附于此位点[44]。以图 2所示石墨烯基底/铜掺杂金属有机框架复合吸附剂为例,其表面通过p轨道-离域π轨道相互作用、离域π轨道-离域π轨道相互作用和范德华力等非取向型作用,吸引甲醛、苯等污染物靠近,进而实现吸附捕集,其不会与任一污染物形成特定排他性吸附结构,因此属于无差别共吸附[45]。
|
图 2 无差别共吸附示意与分子大小对比[45] Fig.2 Schematic diagram of equal co-adsorption and size-contrast of molecules[45] |
一般而言,无差别共吸附方式需要经过“吸附质在吸附剂外扩散流动→吸附质在吸附剂孔内扩散→吸附质被捕集固定”过程[37],其中“吸附质在吸附剂孔内扩散”和“吸附质被捕集固定”通常分别为速率决定步骤和吸附性能决定环节[46],并且由于此方式不针对特定污染物,吸附剂捕集性能的差异主要取决于具体的吸附质。但需要注意的是,相较于CO2/SO2/NOx等小分子而言,室内气态污染物分子(特别是苯及其衍生物)属于大分子(图 2),因此其在吸附剂孔内扩散过程需要重点考虑。
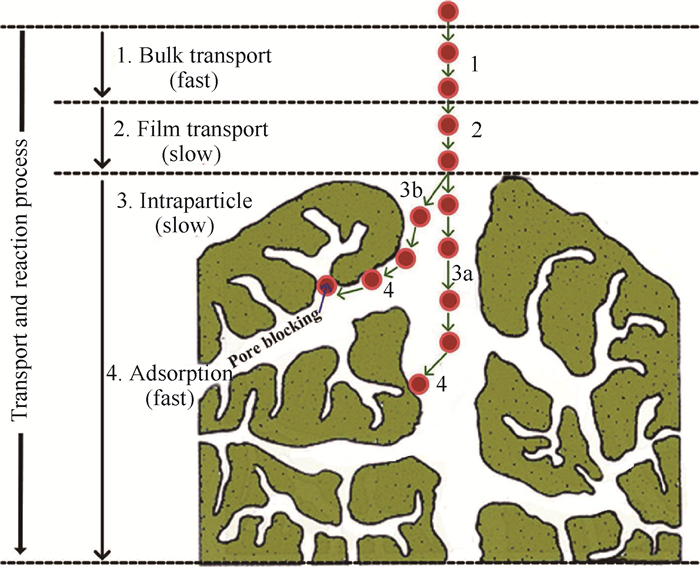
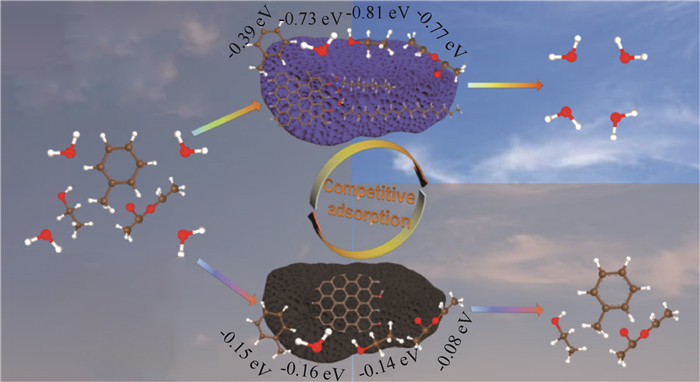
对于上述“吸附质在吸附剂外扩散流动→吸附质在吸附剂孔内扩散→吸附质被捕集固定”过程(图 3)[47],其详细物理化学过程机理如下:1)吸附质在吸附剂外扩散流动。气态污染物在空气中做无规则的分子热运动,其空间浓度分布趋于平均分布,因此其与吸附剂的碰撞结合概率主要取决于吸附剂床层的布置方式与位置;2)吸附质在吸附剂孔内扩散。当气态污染物分子与吸附剂碰撞后,除小部分直接吸附于外表面位点,其余大部分会继续流动、扩散进入吸附剂微孔,并且流动过程会伴随吸附质的捕集固定。孔内扩散过程主要取决于孔径大小/分布与孔道堵塞、气态污染物的分子大小/初始能量以及吸附质捕集固定情况。对于特定吸附剂,其微孔孔径大小/分布等物理结构特性为确定的,因此吸附质分子能否进入微孔内部并被固定是吸附容量的决定性因素。甲醛等小分子容易直接流动进入或被主流动能推动进入吸附剂微孔,而苯系物等大分子的几何结构较为复杂,其与微孔内壁碰撞更为激烈、容易堵塞孔道,进而难以被主流推动进入微孔,因此微孔物理特性是吸附容量的决定性因素之一(图 4)[48];3)吸附质被捕集固定。气态污染物在吸附剂微孔内的流动扩散过程即伴随吸附固定过程,由于常见室内气态污染物之间相互吸引作用较弱、单个吸附位点空间尺寸较小,捕集过程必然伴随着竞争吸附现象,进而影响整体捕集能力(图 5)[49]。
|
图 4 吸附剂微孔特征与吸附性能之间关联[48] Fig.4 Correlation between micropore features and adsorption properties of adsorbents[48] |
|
图 5 吸附表面竞争吸附及其影响示意[49] Fig.5 Schematic diagram of competitive adsorption and its effects over adsorptive surfaces[49] |
根据上述,目前针对无差别共吸附的研究主要在以下2个方面展开:
1) 吸附剂改性及其增强捕集性能的机理。目前常用于无差别共吸附的吸附剂包括活性炭、沸石和金属有机框架等[48]。对于活性炭吸附剂,其吸附活性位点单调(即以碳原子位点为主),因此比表面积(对应单位质量活性炭可暴露的吸附活性位点数量)是影响吸附容量的决定性因素。已有研究采用磷酸(H3PO4)[50]、氢氧化钾(KOH)[51]、氢氧化钠(NaOH)[52]、氯化锌(ZnCl2)[53]和碳酸钾(K2CO3)[54]等活性剂进行活化和表面改性处理,实验结果表明活化/改性处理后,活性炭的比表面积增加1.58~6.17倍、甲醛等污染物的吸附量增加1.46~14.00倍。对于沸石吸附剂,由于空气含湿量对其吸附容量影响较大,常采用氧化石墨烯[55]、硅藻土[56]、铜[57]和银[58]等物质调控沸石中的硅/铝比,增加活性组分,分别用于改善空气湿分在吸附剂表面与污染物争夺吸附位点、污染物与吸附剂的接触捕集强度较弱等问题[59]。对于金属有机框架,其本身具有较大的比表面积和灵活的官能团连接接口,因此常通过加载各类活性组分实现对污染物的超大容量吸附捕集。例如,Eddaoudi等[57]使用溴原子、氨基、异丙醇基团和乙烯基团等修饰MOF-5型金属有机框架化合物,实验结果表明其苯吸附量可达到0.8 g·g-1,远超上述活性炭和沸石的平均吸附容量。Jhung等[58]采用对苯二酸铬修饰MIL-101型金属有机框架化合物,实验结果表明修饰后其苯吸附容量增加3.76倍,最高吸附量可达1.24×10-2 mol·g-1。
2) 多污染物共吸附过程的竞争吸附机理。当前主流观点认为竞争吸附源于不同污染物化学极性/活性之间的差异,其导致污染物靠近吸附剂时诱导产生的瞬时偶极矩强度不同,进而影响污染物与吸附剂的结合强度[61]。例如,Huang等[60]研究了甲苯与氯苯在生物质多孔炭表面的竞争吸附过程,发现由于甲苯的饱和蒸汽压高于氯苯(对应甲苯与吸附剂的结合强度低于氯苯),吸附态甲苯分子会被自由态氯苯分子替换而离开吸附剂表面,即形成竞争吸附。Ma等[61]发现SO2会抑制苯等污染物吸附,其源于SO2具有较高极性,可与吸附剂表面极性基团通过氢键/偶极子-偶极子相互作用等方式优先结合,进而竞争排斥苯等的吸附。Açin等[62]对二甲苯、乙苯和甲苯在复合吸附剂表面的竞争吸附机理进行了研究,发现由于三者具有的电子给体甲基基团数量/位置不同,对吸附剂表面施加的正向诱导作用强度也存在差异,进而造成碳氢-π轨道相互作用、π轨道-π轨道相互作用、范德华力和静电吸引力等效应的综合强度不同,最终出现竞争吸附现象[63-64]。
无差别共吸附方式的优点在于适用场景多元,在多数情况下均能取得较好的吸附净化效果,并且吸附剂微孔物性的调控逻辑单一,可以仅通过改变制备条件而改善微孔结构,进而减少生产成本。但需要注意的是,无差别吸附过程为随机过程,其在特定场景可能会对某些污染物呈现出较差的吸附效果,进而影响整体性能。此外,由于无差别共吸附方式从原理上未考虑潜在的竞争吸附问题,在净化具有复杂组分的室内气态污染物时,必然会出现“高活性组分全被吸附、低活性组分难被捕集”的现象。综合而言,无差别共吸附方式及机理仅适用于室内空气的初步净化,快速实现室内污染物浓度的较大幅度下降,或可用于工厂等对室内空气品质较低的场景。
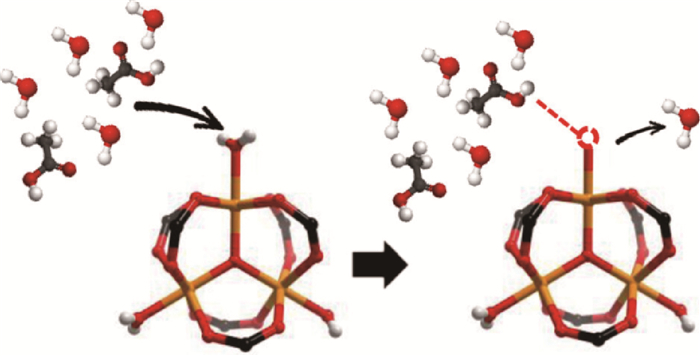
2.1.2 选择性吸附方式选择性吸附方式在选择活性炭等具有优异物理结构的材料作为吸附剂基底的基础上,通过复合其他活性材料、调控/载入表面官能团或修饰/改性原有特定位点等方式使吸附位点呈现单一极性化学取向,进而仅针对某个或某类气态污染物分子呈现出极强的物理/化学吸引作用,而对其他污染物呈中性乃至排斥作用[65-66]。如图 6所示,当乙酸分子靠近MIL型金属有机框架化合物的开放金属位点时,由于两者倾向于化合生成更稳定的乙酸盐形态,原有物理吸附于该位点的水分子被排斥离开,即整体呈现选择性吸附特征[67]。
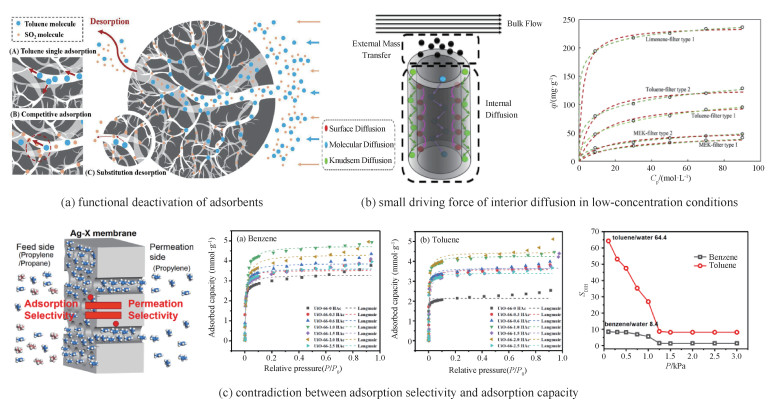
从吸附的物理化学过程而言,选择性吸附方式与上述的无差别共吸附方式并无区别。然而,由于选择性吸附方式的目标仅为个别室内气态污染物,因此其在吸附净化过程中会出现3个难以回避的问题[68-71]:1)吸附剂中毒。此处的吸附剂中毒类似于吸附剂功能性失活的概念,是指吸附剂表面总会优先被高活性组分占据,而使得低活性的目标组分难以被捕集。如图 7(a)所示,SO2分子优先占据吸附位点后会形成硫酸盐或亚硫酸盐,该位点无法再吸附甲苯分子,导致吸附剂中毒失活;2)吸附自发性较弱。室内气态污染物的总浓度(即分压)较低,其难以自发扩散进入吸附剂被捕集,而单一目标组分的浓度(即单组分分压)更是远低于总浓度,因此其流动-扩散-吸附过程的自发性会更弱、更难以被吸附剂捕集。如图 7(b)所示,当甲苯浓度较低时,其扩散进入过滤器孔隙的速率也较低,对应的稳定态平衡吸附量也较小,即吸附潜力无法全部发挥;3)吸附选择性-吸附量矛盾。对于低浓度、活性相近的室内气态污染物而言,吸附选择程度越高,所捕集的吸附质中目标组分的占比随之越高,但此吸附质的捕集量却会随之降低。如图 7(c)所示,当甲苯、苯的组分分压增加时,其吸附量随之提高,但甲苯/水、苯/水的吸附选择性随之降低。
|
图 7 选择性吸附过程中的3个问题[68-71] Fig.7 Three unavoidable problems during processes of selective adsorption[68-71] |
针对选择性吸附方式的目标以及上述3个无法回避的问题,目前常用的策略有以下2种:1)化学性质方面-负载活性基团/官能团。针对甲醛、苯等气态污染物的化学性质,例如甲醛易与碱性基团反应结合、苯环具有亲电取代反应特性,制备具有特殊官能结构的吸附剂,通过特定化学反应过程实现高效选择性吸附。此方法优点在于吸附选择性强、定向捕集精准,但存在制备过程复杂、吸附剂难以循环使用等问题;2)物理性质方面-调节微孔结构。根据目标污染物的分子尺寸和分子自由程,通过设计吸附剂微孔结构,特别是需要平衡孔道取向-孔径大小分布-孔容总量与比表面积大小之间的关系,实现特定尺寸以下的气态污染物分子可扩散进入吸附剂内孔,其他尺寸的气态污染物分子仅能流过或固定于吸附剂外表面。此方法优势在于吸附量较大且基本吸附特性不受改性影响,但其针对特定尺寸以下的污染物分子,因此选择吸附性较差(例如能透过苯分子的孔径,也基本可使甲醛分子通过)。
当前室内气态污染物吸附净化研究中常用的选择性吸附强化具体手段如表 1所示[72-81]。其中,对碳基吸附剂的主要改性方式为使用碱性溶液(如氢氧化钾)浸渍。一方面,浸渍碱性溶液可以避免碳材料活化过程中生成焦油,还可降低活化所需温度并加速氮、氢等非碳元素移除,进而加快微孔生成、增大吸附剂比表面积[72];另一方面,在浸渍碱性溶液后,碳材料活化过程中会生成对应的碱金属(例如钾),这些碱金属具有极强的还原性,可在污染物吸附过程中提供活性电子,进而促进π轨道相互作用等效应[73]。
|
|
表 1 室内空气选择性吸附净化常用手段及效果 Table 1 Typical methods and their effects on selective adsorption for indoor air purification |
选择性吸附方式的优势为定向捕集准确率高,可实现对特定室内气态污染物组分的高效吸附,进而完成特殊场景下的室内空气净化。同时,由于此方法较强的选择特性,可作为下述“催化氧化/还原吸附”方法的前置吸附环节,进而增强吸附净化效率。值得注意的是,由于大多数室内场景中的气态污染物浓度较低(甚至极低)、相应组分的分压也较小,在实际场景中选择性吸附方式的单一表观净化效果较差,此方法往往需要与上述“无差别共吸附”方式进行联用,即先进行“无差别共吸附”净化以达到污染物整体浓度大幅下降,再进行“选择性吸附”精细处理净化特定污染物,最终实现全范围高效吸附净化。
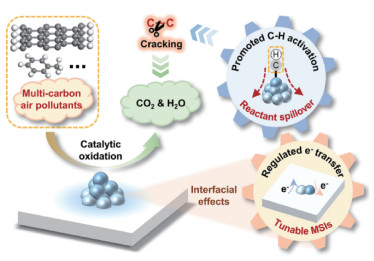
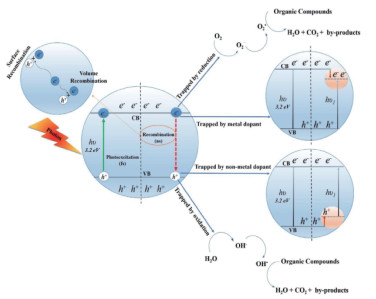
2.2 催化氧化/还原吸附催化氧化/还原吸附是指采用过渡金属氧化物等高活性材料作为预吸附-催化基底,使特定室内气态污染物被捕集后通过氧化还原反应转化为CO2/H2O等无害气态污染物排入大气,或形成更易捕集的其他形态进行强化吸附(图 8)[82-84]。在目前的室内气态污染物催化处理技术路线中,光催化是最常用的路线。相较于其他技术路线,光催化不仅具有最广泛的潜在适用场景,有较大潜力应用于特定自然光或人工照明的室内场景,而且具有较为广泛的催化净化范围,可对甲醛、苯和甲苯等多种污染物实现最高100 % 的催化净化效果。需要注意的是,当前光催化的基本原理建立于二氧化钛(TiO2)在光照下将H2O分解为氧气(O2)的过程中可产生自由基,自由基可与部分大分子结合并将其逐步分解为无机小分子(如CO2),其延伸用于室内气态污染物净化过程(图 9)[85]:首先,具有光敏特性的半导体催化材料(如TiO2、CeO2)受光照会产生大量电子空穴对,用于提供氧化还原反应基料;接着,这些电子空穴对可将O2和H2O分别转化为自由基O*和OH*,分别用作污染物氧化和还原过程的配料;最后,不同的室内气态污染物分别与O*和OH*不断结合生成H2O、CO2以及副产品,其中H2O/CO2会排入大气,副产品则会被深度固定捕集或进一步分解。
|
图 8 室内气态污染物催化氧化/还原过程示意[82-84] Fig.8 Schematic diagram of catalytic oxidation/reduction processes for indoor gaseous pollutants[82-84] |
|
图 9 光催化用于室内气态污染物净化过程原理[85] Fig.9 Mechanism of photocatalysis for purification processes of indoor gaseous pollutants[85] |
针对室内气态污染物光催化净化,目前最常见目标污染物种类为苯系污染物,次要常见目标污染物为甲醛:
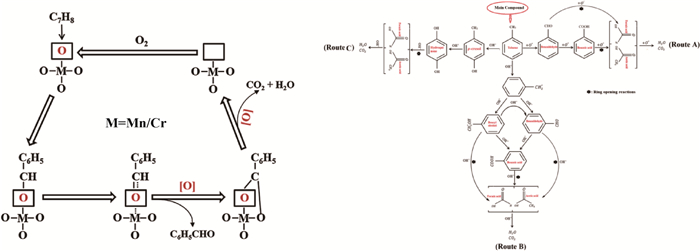
1) 对于苯系污染物,其光催化转化的基本过程以图 10所示的甲苯(C6H5CH3)分解为例[86-87]。
|
图 10 苯系污染物光催化净化过程示意[86-87] Fig.10 Schematic diagram of photocatalytic purification processes of benzene-series pollutants[86-87] |
a) 路线1(图 10中左列与右列的Route A)。首先,C6H5CH3中甲基基团的两个H原子与O2分解产生的O*结合生成H2O,C6H5CH3的剩余部分(C6H6-CH)与O*结合生成苯甲醛(C6H5CHO)。随后,C6H5CHO继续与O*结合生成苯甲酸(C6H5COOH),C6H5COOH与O*发生开环反应,生成甲酸(HCOOH)和乙酸(CH3COOH)。最后,HCOOH和CH3COOH与O*反应生成H2O与CO2;
b) 路线2(图 10中右列的Route B)。首先,C6H5CH3与H2O分解产生的OH*结合生成甲苯自由基(C6H5CH2*)和H2O。随后,C6H5CH2*与OH*反应,生成苯甲醇自由基(C6H5CH2*OH)或C6H5CHO,C6H5CH2*OH与C6H5CHO分别与OH*继续反应,均生成C6H5COOH。最后,C6H5COOH与OH*发生开环反应生成HCOOH和CH3COOH,两者最终与OH*反应生成H2O与CO2;
c) 路线3(图 10中右列的Route C)。首先,C6H5CH3与OH*结合,生成对甲酚(CH3C6H4OH)和H2O。随后,CH3C6H4OH继续与OH*反应,生成对苯二酚(HOC6H4OH)。最后,HOC6H4OH与OH*发生开环反应生成HCOOH和CH3COOH,两者最终与OH*反应生成H2O与CO2。
由上述机理可知,自由基O*和OH*是苯系污染物光催化转化过程的最关键配料,而自由基的产生速率、数量和位置等参数是影响光催化的核心因素。在目前的研究中,加快自由基生成的方法有引入臭氧(O3)作为O*生成原料、使用稀土金属/过渡金属氧化物产生氧空位以及使用不同强度/波长的光照。其中,引入O3的方法在原理/实践上均较为简单,但O3可能会泄露扩散进入室内造成二次污染,而使用更适合光催化强度/波长的光照则可能不符合实际室内光照环境,因此调控高活性金属氧化物以更快地产生更多氧空位是当前最主流的方法。不同调控/强化条件下苯系污染物的光催化净化效果如表 2所示[31, 88-110]。
|
|
表 2 不同调控/强化条件下苯系污染物光催化净化效果 Table 2 Photocatalytic purification effects for benzene-series pollutants under different regulation/enhancement conditions |
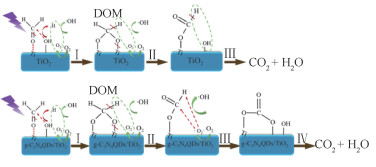
2) 对于甲醛,其光催化氧化的过程为“甲醛分子吸附→形成二甲醛形态→转化为甲酸盐形态→变为碳酸盐形态→生成CO2/H2O”(图 11)[111-113]。如图 11所示,在TiO2表面产生氢氧根自由基OH*是启动甲醛光催化转化的第一步,因此自由基产生速度/数量/位置等参数也是影响甲醛光催化转化的决定性条件。与苯系污染物不同,甲醛属于唯一结构小分子,其光催化转化过程较为固定且单一,因此相关研究主要集中于自由基产生强化和转化过程能垒削弱。对于自由基产生强化的研究,目前主要集中在TiO2等催化材料制备及其性能表征,而转化过程能垒削弱的相关研究主要围绕于动力学特征(温度、压强等)、过渡态能垒与转化路线等[114]。甲醛光催化转化过程强化方法与效果如表 3所示[31, 96, 111, 114-130]。
|
图 11 甲醛光催化净化过程示意[111-113] Fig.11 Schematic diagram of photocatalytic purification processes of formaldehyde[111-113] |
|
|
表 3 甲醛光催化净化过程强化方法与效果 Table 3 Enhancement methods and their effects for photocatalytic purification processes of formaldehyde |
催化氧化/还原吸附方式的定向净化效率极高,对特定场景下的特定污染物最高达到近100 % 的净化效率,并且催化吸附净化后基本不会再出现二次污染,实现了真正意义上的绿色环保。然而,相较于上述“物理/化学吸附”方式,此方式的室内适用条件较为苛刻,绝大部分室内光照条件不满足光催化转化所需强度,更不必说夜晚、阴天等光照条件极差的情况,因此当前实验室制备的光敏催化剂基本无法在近零能耗下实现室内空气长效净化。此外,目前催化氧化/还原吸附方法仅能实现甲醛/苯/甲苯等少数室内污染物的净化,对于污染物组分复杂的实际室内场景无能为力,并且其他污染物对甲醛/苯/甲苯等光催化转化的影响未知。
综合而言,“物理/化学吸附”方式以多孔材料的微孔物性调控为基础、以表面位点的化学吸附特性设计为核心,构建以强化分子间相互作用和特定化合反应为主要吸附过程的可逆式室内气态污染物捕集机制,其优点为可捕集的污染物种类多、可实现长效净化以及可构建循环吸附体系,缺点在于捕集能力较为有限、吸附产物易被再释放以及多组分共吸附作用机理较复杂。“催化氧化/还原吸附”方式以强化自由基产生性能为出发点、以提高污染物定向催化转化为CO2/H2O的选择性为目标,基于TiO2光催化分解H2O可产生高活性自由基用于室内气态污染物分解自反应,此方式的优点在于定向净化效率高、催化转化的最终产物无害且反应产物无法再重新生成污染物,相应的缺点在于室内适用条件极其严格、无法实现长效净化且在实际复杂室内场景下净化效果不明。
3 室内气态污染物吸附技术 3.1 负压过滤吸附技术负压过滤吸附技术主要基于“物理/化学吸附”机理,以商用工业多孔材料作为吸附介质,通过空气压缩机等机械装置产生局部负压,进而实现室内空气定向流过吸附床层完成吸附过滤净化(图 12)[131-134]。根据上述工作机理可知,多孔吸附材料性能/吸附床层布置、局部负压产生方式/空气过滤范围与速率是影响负压过滤吸附技术的决定性因素:
|
图 12 负压过滤吸附技术示意图[131-134] Fig.12 Schematic diagram for negative-pressure filtration adsorption technology[131-134] |
1) 在吸附材料及其布置方面[135],目前最常采用的是基于活性炭吸附材料的滤网方案。其中,活性炭主要选取高硬度、高强度和以微孔孔道为主的类型,当前以经过高温、活化以及碳化处理的椰维炭为最佳[136],除了具备比表面积大、微孔体积占比高等活性炭的常规特性,其还具有微孔分布均匀、吸附速率快以及杂质少等优点。而滤网方案主要用于室内空气预处理,在气态污染物到达活性炭吸附床层前对颗粒物等固态污染物进行捕集,其捕集性能与滤网网孔密度呈正相关、与颗粒物阻塞网孔概率呈负相关。
2) 在空气过滤速度控制方面[137],目前主要选择可变速风机作为空气定向流动过滤的动力来源,并辅以精细流道设计,可实现较高的空气过滤净化效率。此外,为进一步增强空气过滤效率,可在风机与滤网之间增设高压静电场,使颗粒物带电并通过静电作用吸附至经特殊处理而永久带电的滤芯/滤网处,进而为实现气态污染物长效吸附净化提供更多时间和空间。但需要注意的是,高压放电过程容易产生O3,形成二次污染。
负压过滤吸附技术属于主动式室内空气净化方案,其优点在于使用条件简单,几乎可适用于所有室内场景,并且对室内所有污染物均具有较好的净化效果,特别是其对固态污染物和气态污染物分段净化,为后续吸附性能进一步提升提供了较好的基础。然而,目前采用负压过滤吸附技术的空气净化器仅能对其周围产生一定的净化效果;当室内空间较大时,空气净化速率和效果均难以令人满意。此外,为强化颗粒物过滤而采用的高压静电集尘,容易产生O3排放,故静电驻极方案更有前景,此处不作详细讨论。
3.2 催化吸附净化技术催化吸附捕集技术基于上述“催化氧化/还原吸附”原理,以纳米级TiO2光触媒作为催化核心材料,通过将其涂抹于惰性材料表面形成光敏薄膜,在特定波长光照下产生自由基用以降解室内气态有害污染物、杀灭细菌(图 13)[138-139]。
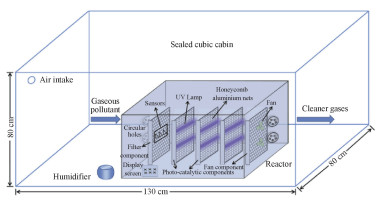
|
图 13 催化吸附净化技术原理示意图[138-139] Fig.13 Schematic diagram of catalytic adsorptive purification technology[138-139] |
由上述机理可知,工业商用光触媒的本征性能、光触媒/基底复合物-光敏薄膜表观性能以及空气定向流过光触媒表面的特征是影响催化吸附捕集技术的核心因素。其中,空气定向流过光触媒表面的特征与上述“负压过滤吸附技术”中的相关技术细节相似,此处不再赘述,因此光触媒的本征性能与光敏薄膜的表观性能是催化吸附捕集技术提升的要点:
1) 对于光触媒的本征性能而言[140],在当前TiO2光催化分解H2O机理已完全掌握的情况下,需要对TiO2本征物理化学性质进一步研究,以增强自由基的产生性能。在TiO2的物理性质方面(表 4)[141-149],目前研究分别从结构特征和反应动力学特征2方面出发进行精细调控。
|
|
表 4 光触媒(TiO2)物理性质调控 Table 4 Regulation of physical features of photocatalyst (TiO2) |
a) 在结构特征方面,制备粒径更小、比表面积更大、微孔结构更合理、晶型纯度更高、表面吸附位点分布更均匀和表面吸附取向更单一的结构是提高光触媒性能的研究重点。例如,Wang等[134]发现,当SiO2/TiO2复合催化剂的粒径从1.4×10-8 m增大到1.67×10-8 m时,其光电流从0.032 A·m-2减小至0.022 A·m-2。Liu等[138]证明比表面积与氧空穴的生成速率有关,当TiO2比表面积从36.6 m2·g-1增加至130.7 m2·g-1时,其表面光电流大幅增加,相应的光催化生成氨的速率从3.8×10-5 mol·g-1·h-1上升到1.16×10-4 mol·g-1·h-1。Wang等[140]对比甲硝哒唑在TiO2(1 0 1)和(0 0 1)表面光催化分解过程,发现后者的速率决定能垒是前者的2.74倍,从而证明(1 0 1)表面的催化活性更强。
b) 在反应动力学特征方面,建立更快速高效的吸附质-吸附剂碰撞模型、调控合适的反应温度/压力等是强化光触媒反应速率的常用方法。例如,Safri等[141]分别采用拟一阶和拟二阶动力学模型研究甲基蓝在二氧化钛/碳复合催化剂表面的吸附过程,发现拟二阶动力学模型更符合实际吸附过程,其决定系数R2最高可达0.998。Trzeciak等[142]研究乙烯在二氧化钛/泡沫镍复合催化剂表面的光催化分解特性,发现当温度上升时,乙烯的分解率先增加后减少,在100 ℃达到峰值(27.05 % ±1.05 %),此现象源于乙烯室内扩散速率增加,附着于催化剂表面的分子数量减少。Li等[143]研究CO2在二氧化钛-碳化钛/石墨相氮化碳复合催化剂表面光催化分解为CO过程,发现随压力上升,CO2分解速率上升并最终达到稳定平衡态,其反映了反应物吸附于吸附剂表面和产物离开吸附剂表面之间的动态平衡关系。
在TiO2的化学性质方面(表 2、表 3),掺杂高活性过渡/稀土金属、优化自由基产生路线/降低产生能垒、调控形成更合理的吸附质-吸附剂结合模型以及减少光触媒非稳定性失活概率等是增强光触媒化学特性的方法。
2) 对于光敏薄膜的表观性能而言[150-152],在高活性光触媒材料与高稳定性基底材料的基础上,光敏薄膜的性能主要由其制备工艺及对应的结构决定。一方面,光敏薄膜需要保持稳定的结构,在强光照、不同风向空气流动等条件下均能保持原有结构以实现长效循环催化,这需要基底材料具有一定的致密性,防止出现内应力导致薄膜结构破坏;另一方面,CO2/H2O等催化反应产物需尽快离开薄膜表面,以实现催化吸附净化可以循环进行,这便要求薄膜结构具有尽可能多的微孔,特别是基底材料内部具有较多空隙。上述两点要求具有一定的矛盾性,因此设计并调控光敏薄膜的多组分相对结构特征是增强光敏薄膜表观性能的必由之路。
催化吸附净化技术也属于主动式室内空气净化方案,其优点在于可将室内气态污染物直接转化为无害产物,实现了真正意义上的绿色环保,并且针对特定工况下单一气态污染物可实现极高的催化吸附净化效率。然而,由于现有催化反应路径较复杂、自由基产生速率/数量难以有效控制并且室内照明光源难以满足催化反应的需要(需另外加设专用紫外光源),目前商用光催化空气净化装置在典型室内场景下的空气净化效率较低,同时会产生剧毒中间产物。此外,催化吸附净化技术往往需要紫外光源用以激励辅助,降低了其适用范围。
3.3 生物修复净化技术生物修复净化技术主要基于植物(或植物组织)及其叶围/根围微生物对周围气态污染物的生物提取、生物挥发、生物降解、生物固定、根围降解和根围过滤过程(图 14)[153-155]。生物提取过程采用超富集植物作为载体,用于吸收污染物并富集于叶围区域,并可以根据需要将植物富集区域人工采集并煅烧以实现彻底净化[156]。对于生物挥发过程,气态污染物会在特定植物的叶围区域被降解转化为毒性更低的化合物(例如CO2),并通过植物的气孔蒸发进入大气环境[157]。生物降解又称为生物转化,在此过程中室内气态污染物会被转化并吸收进入植物组织结构,进而通过漆酶/脱卤素酶/硝基还原酶等的新陈代谢作用后进入大气或土壤等[158]。生物固定一般发生于植物的根围区域,通过根部细胞的细胞壁木质素或植物的腐殖质吸收气态污染物,将其沉积于不溶性的化合物中并最终富集于根围区域[159]。根围降解和根围过滤的基本物理化学过程与上述其他过程基本类似,仅在生化过程方面有一定不同[158],此处不再赘述。生物修复净化技术对室内气态污染物的净化作用如表 5所示[155-170]。
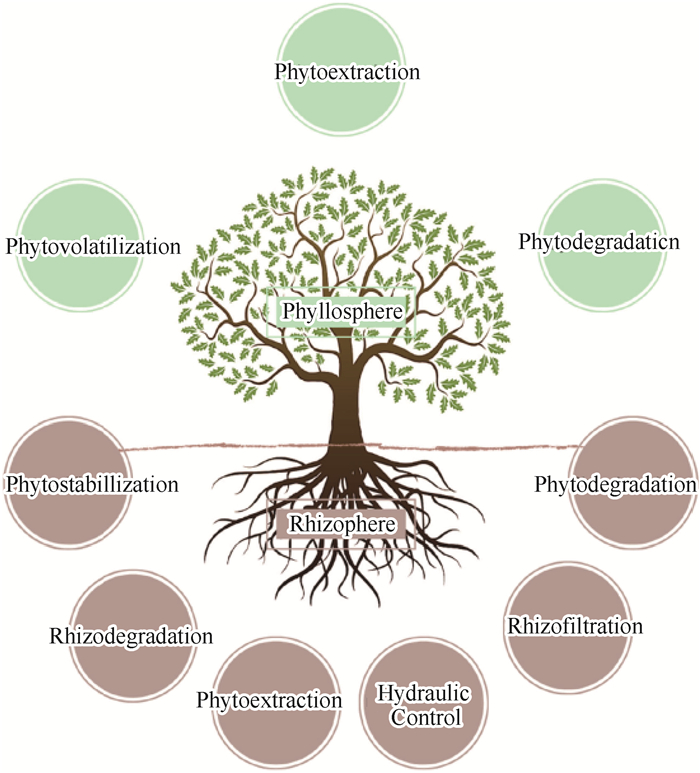
|
图 14 生物修复净化技术示意[153-155] Fig.14 Schematic diagram of phytoremediation purification technology[153-155] |
|
|
表 5 生物修复净化技术对室内气态污染物的净化作用 Table 5 Purification effects of phytoremediation purification technologies on indoor gaseous pollutants |
综上,生物(植物及其共生微生物)净化室内气态污染物的基本过程和环节如下(以家庭常见盆栽绿萝、芦荟和君子兰等净化甲醛为例[171-172]):
a) 植物吸收气态甲醛。植物叶片通过气孔与环境气体直接进行交换,因此单位时间气态甲醛的吸收量与单位面积叶片气孔数量呈正相关[173]。一般而言,肉质植物由于其叶片内部的叶肉组织较为丰富,会挤压上下表皮内侧形成的腔隙(气室),进而影响其与内外气体交换(包括吸收甲醛);而对于具有针形/硬质/革质叶片的植物,其表皮较为致密并且侧壁表面细胞相互嵌合、紧密相连,因此气孔孔径相对较小,抑制甲醛吸收[174]。同时,叶龄对甲醛吸收也较为重要,幼叶为减少水分损失而降低气孔导度(气孔张开程度),导致甲醛吸收量降低,而成熟叶片所需光合作用强度较高,会增加气孔导度以增强呼吸作用,进而增加甲醛吸收量[175]。此外,植物的甲醛吸收能力也与光强、光合作用强度有关。一般而言,光照越强烈,植物体内叶绿体生成生物量速率(光合作用)越快,所需的CO2量也越多,进而需要增加气孔导度、增强叶片内外气体交换速率,最终提高甲醛吸收量[176]。
b) 植物体内代谢甲醛。气态甲醛通过叶片气孔进入植物后,会进一步通过根围溶液转移至植物根部。在此转移过程中,叶片角质层的渗透程度是影响植物吸收、转移甲醛速率的决定性因素[177]。当甲醛被吸收进入植物组织后,其会通过以酶促反应为主的耗散机制和氧化还原反应进行分解,最终分解产物为CO2和H2O[178]。Su等[175]发现,调节环境压力可以增加活性氧的产生速率,进而提高植物体内甲醛氧化还原分解速率。在上述酶促反应和氧化还原过程中,植物为避免甲醛中毒,会通过卡尔文循环利用谷胱甘肽将甲醛转化为有机酸(例如甲酸)、糖和氨基酸[179],并进一步利用甲酸脱氢酶将甲酸氧化为CO2[178]。此外,植物在进行单碳代谢过程中也会产生甲醛,其作为中间产物可被5, 10-亚甲基四氢叶酸直接氧化为CO2和H2O,进而避免甲醛中毒[180]。
c) 根围微生物分解甲醛。在甲醛持续释放扩散的场景中,当植物叶片处于幼龄期时,其对甲醛吸收能力较弱,此时主要通过植物根系和根围土壤/微生物吸收甲醛[181]。Xu等[182]发现,绿藻根系不仅可以直接吸收甲醛,而且会在吸收过程中产生分泌物,进一步增强甲醛的生物降解和生物过滤速率。同时,Seco等[179]通过对比实验发现,对于某些特定植物,当其根围附近没有微生物时,相应的甲醛净化率为0,而当人工增加根围微生物组群后,其甲醛净化率高达100 %。此外,Schmitz等[180]发现,甲醛可通过叶片气孔直接向下转移至根围。Haslam等[181]向植物根围添加假丝酵母等微生物,发现其会显著提高甲醛从气孔转移到根围的速率,并且整体净化率最高达90 %、甲醛净化速率最高达1.35×10-4 g·h-1。
生物修复净化技术一般属于被动式空气净化方案,其优点在于在简单条件下即可实现长效净化,并且可对所有污染物产生一定的净化作用。此外,此技术基本可实现永久空气净化,即已被吸收/吸附的污染物会固定于植物/微生物表面或内部,不会再以原形态返回大气。然而需要注意的是,生物修复净化技术的净化效率完全取决于植物/微生物本身的代谢速率,因此在通常情况下净化速度极慢,无法满足室内品质要求。同时,培育更高净化效率的植物/微生物所需的经济/时间成本较高,并且其针对特定场景下特定气态污染物的选择净化性能也难以调控。
综上所述,“负压过滤吸附技术”以“物理/化学吸附”机理为基础,通过构建特定负压区域,可定向实现滤网捕集颗粒物-多孔材料吸附气态污染物,其优点在于净化速率高、捕集污染物范围广,但缺点在于其仅能净化周围空气并且可能产生O3等二次污染物。“催化吸附净化技术”以“催化氧化/还原吸附”机理为核心,采用光敏薄膜在特定波长光照下产生自由基对气态污染物进行催化转化,其在特定条件下对特定污染物可实现极高的净化效率,但当前商用光催化净化装置的净化效率极低,需进一步优化催化材料/路径。“生物修复净化技术”以特定植物/微生物对室内空气污染物的生化吸收过程为理论框架,选取/培育植物/微生物以特定生物过程对相应污染物进行处理净化,其优点在于长效绿色持久净化,且从根本上杜绝产生有害中间体或产物,但同时也存在净化效率低、培育成本高等问题。
4 结语本文对甲醛、苯等常见室内气态污染物的吸附机理和技术进行了总结,吸附机理主要有“物理/化学吸附”和“催化氧化/还原吸附”两类,吸附技术包括“负压过滤吸附技术”“催化吸附净化技术”“生物修复净化技术”三种。通过文献调研发现,当前研究缺乏从基本机理入手开发更有效的吸附剂,亦没有从现有吸附技术存在的问题出发倒推吸附剂/吸附过程改性强化的应有措施。因此,本文对这些吸附机理和技术进行了详细汇总和分析,并对不同机理/技术的特点、适用条件和改进方向进行了概括与讨论,为后续室内空气吸附净化机理和技术研究提供了良好参考。
为使室内空气吸附净化性能更上一层楼,在后续的研究中可先限定室内场景及其污染物种类/形态/含量等特征,然后根据这些特征选取合适的吸附机理/技术,或将上述机理/技术组合使用(例如采用碳基吸附剂进行无差别共吸附后,将其埋入植物根茎处,使污染物通过生物固定或根围降解等方式被生物净化;制备双层吸附剂,其中一侧通过无差别共吸附或选择性吸附捕集污染物,另一侧使用光照将污染物快速转化为CO2/H2O,实现循环吸附捕集)以制备吸附性能更优良的吸附剂、布置吸附过程更合理的吸附床层等,为实现复杂场景室内空气高效吸附净化提供必要基础。
| [1] |
丁洁, 高超, 王亚东, 等. 常见室内污染物吸附材料研究进展[J]. 材料科学与工程学报, 2022, 40(5): 901-908. DING J, GAO C, WANG Y D, et al. Research progress of adsorption materials for common indoor pollutants[J]. Journal of Materials Science & Engineering, 2022, 40(5): 901-908. |
| [2] |
王智超, 邓高峰, 王志勇, 等. 建筑室内污染物控制技术研究[J]. 建筑科学, 2013, 29(10): 63-70. WANG Z C, DENG G F, WANG Z Y, et al. Research of indoor pollutant control technology[J]. Building Science, 2013, 29(10): 63-70. DOI:10.3969/j.issn.1002-8528.2013.10.010 |
| [3] |
李灏阳, 夏辉华. 室内空气污染控制技术现状与趋势[J]. 建筑热能通风空调, 2017, 36(2): 86-89. LI H Y, XIA H H. Trend of indoor air pollution control technology[J]. Building Energy & Environment, 2017, 36(2): 86-89. DOI:10.3969/j.issn.1003-0344.2017.02.021 |
| [4] |
CHANG T, WANG J H, LU J Q, et al. Evaluation of indoor air pollution during the decorating process and inhalation health risks in Xi'an, China: A case study[J]. Aerosol and Air Quality Research, 2019, 19(4): 854-864. DOI:10.4209/aaqr.2018.07.0261 |
| [5] |
FU N, WEI P, JIA Y B, et al. Indoor volatile organic compounds in densely occupied education buildings of four universities: Target list, concentration levels and correlation analysis[J]. Building and Environment, 2021, 191: 107599. DOI:10.1016/j.buildenv.2021.107599 |
| [6] |
KOZIELSKA B, KALETA D. Assessment of indoor benzene and its alkyl derivatives concentrations in offices belonging to University of Technology (Poland)[J]. Atmosphere, 2021, 12(1): 51. |
| [7] |
CHOI H, KIM H, KIM T. Long-term simulation for predicting indoor air pollutant concentration considering pollutant distribution based on concept of CRPS index[J]. Building Simulation, 2019, 12(6): 1131-1140. DOI:10.1007/s12273-019-0550-4 |
| [8] |
ARDEH S A, KHALOO S S, GHOLAMNIA R, et al. Assessment of indoor air pollutant concentrations and emissions from natural gas cooking burners in residential buildings in Tehran, Iran[J]. Air Quality, Atmosp here & Health, 2020, 13(4): 409-420. |
| [9] |
FAZLI T, DONG X Y, FU J S, et al. Predicting U.S. residential building energy use and indoor pollutant exposures in the mid-21st century[J]. Environmental Science & Technology, 2021, 55(5): 3219-3228. |
| [10] |
VAN TRAN V, PARK D, LEE Y C. Indoor air pollution, related human diseases, and recent trends in the control and improvement of indoor air quality[J]. International Journal of Environmental Research and Public Health, 2020, 17(8): 2927. DOI:10.3390/ijerph17082927 |
| [11] |
ALI M U, YU Y M, YOUSAF B, et al. Health impacts of indoor air pollution from household solid fuel on children and women[J]. J ournal of Hazard ous Mater ials, 2021, 416: 126127. DOI:10.1016/j.jhazmat.2021.126127 |
| [12] |
KAMAL R, SRIVASTAVA A K, KESAVACHANDRAN C N, et al. Chronic obstructive pulmonary disease (COPD) in women due to indoor biomass burning: A meta analysis[J]. International Journal of Environmental Health Research, 2022, 32(6): 1403-1417. DOI:10.1080/09603123.2021.1887460 |
| [13] |
KUMAR P, SINGH A B, ARORA T, et al. Critical review on emerging health effects associated with the indoor air quality and its sustainable management[J]. Science of t he Total Environment, 2023, 872: 162163. DOI:10.1016/j.scitotenv.2023.162163 |
| [14] |
CHEN Y C, GUI Z H, BAO W W, et al. Chronic exposure to indoor air pollutants in association with attention-deficit/hyperactivity disorder symptoms in Chinese schoolchildren: A cross-sectional study[J]. NeuroToxicology, 2023, 94: 182-190. DOI:10.1016/j.neuro.2022.12.003 |
| [15] |
LI Y G, CHEN Z D. A balance-point method for assessing the effect of natural ventilation on indoor particle concentrations[J]. Atmospheric Environmen t, 2003, 37(30): 4277-4285. DOI:10.1016/S1352-2310(03)00527-2 |
| [16] |
CHALLONER A, GILL L. Indoor/outdoor air pollution relationships in ten commercial buildings: PM2.5 and NO2[J]. Building and Environment, 2014, 80: 159-173. DOI:10.1016/j.buildenv.2014.05.032 |
| [17] |
JI W J, CHEN C, ZHAO B. A comparative study of the effects of ventilation-purification strategies on air quality and energy consumption in Beijing, China[J]. Building Simulation, 2021, 14(3): 813-825. DOI:10.1007/s12273-020-0694-2 |
| [18] |
SHENG Y, FANG L, SUN Y X. An experimental evaluation on air purification performance of Clean-Air Heat Pump (CAHP) air cleaner[J]. Building and Environment, 2018, 127: 69-76. DOI:10.1016/j.buildenv.2017.10.039 |
| [19] |
SHIUE A, HU S C, TSENG C H, et al. Verification of air cleaner on-site modeling for PM2.5 and TVOC purification in a full-scale indoor air quality laboratory[J]. Atmospher ic Pollution Research, 2019, 10(1): 209-218. DOI:10.1016/j.apr.2018.07.008 |
| [20] |
CABOVSKÁ B, BEKÖ G, TELI D, et al. Ventilation strategies and indoor air quality in Swedish primary school classrooms[J]. Building and Environment, 2022, 226: 109744. DOI:10.1016/j.buildenv.2022.109744 |
| [21] |
PACITTO A, AMATO F, MORENO T, et al. Effect of ventilation strategies and air purifiers on the children's exposure to airborne particles and gaseous pollutants in school gyms[J]. Science of the Total Environ ment, 2020, 712: 135673. DOI:10.1016/j.scitotenv.2019.135673 |
| [22] |
ZHANG T H, ZHANG Y X, LI A Q, et al. Study on the kinetic characteristics of indoor air pollutants removal by ventilation[J]. Building and Environment, 2022, 207: 108535. DOI:10.1016/j.buildenv.2021.108535 |
| [23] |
GUO M, PEI X Q, MO F F, et al. Formaldehyde concentration and its influencing factors in residential homes after decoration at Hangzhou, China[J]. Journal of Environmental Sciences, 2013, 25(5): 908-915. DOI:10.1016/S1001-0742(12)60170-3 |
| [24] |
CHU C R, YANG K J. Transport process of outdoor particulate matter into naturally ventilated buildings[J]. Building and Environment, 2022, 207: 108424. DOI:10.1016/j.buildenv.2021.108424 |
| [25] |
MOHAMMADI M, CALAUTIT J. Impact of ventilation strategy on the transmission of outdoor pollutants into indoor environment using CFD[J]. Sustainability, 2021, 13(18): 10343. DOI:10.3390/su131810343 |
| [26] |
ZHANG C R, YAO L, YANG Z, et al. Graphene oxide-modified polyacrylonitrile nanofibrous membranes for efficient air filtration[J]. ACS Applied Nano Materials, 2019, 2(6): 3916-3924. DOI:10.1021/acsanm.9b00806 |
| [27] |
ZOU N, NIE Q, ZHANG X R, et al. Electrothermal regeneration by Joule heat effect on carbon cloth based MnO2 catalyst for long-term formaldehyde removal[J]. Chemical Engineering Journal, 2019, 357: 1-10. DOI:10.1016/j.cej.2018.09.117 |
| [28] |
CHEN Q W, TIAN E Z, LUO Z Y, et al. Adsorption film with sub-milli-interface morphologies via direct ink writing for indoor formaldehyde removal[J]. J ournal of Hazard ous Mater ials, 2022, 427: 128190. DOI:10.1016/j.jhazmat.2021.128190 |
| [29] |
LIU R, LIANG M, XU J F, et al. Preparation of a novel formaldehyde-free impregnated decorative paper containing MnO2 nanoparticles for highly efficient formaldehyde removal[J]. ACS Applied Materials & Interfaces, 2023, 15(29): 34941-34955. |
| [30] |
CHEN Q W, XIAO R, LEI X, et al. Experimental and modeling investigations on the adsorption behaviors of indoor volatile organic compounds in an in-situ thermally regenerated adsorption-board module[J]. Build ing and Environ ment, 2021, 203: 108065. DOI:10.1016/j.buildenv.2021.108065 |
| [31] |
WANG W J, LIN F W, AN T C, et al. Photocatalytic mineralization of indoor VOC mixtures over unique ternary TiO2/C/MnO2 with high adsorption selectivity[J]. Chemical Engineering Journal, 2021, 425: 131678. DOI:10.1016/j.cej.2021.131678 |
| [32] |
NOREÑA-CARO D, ÁLVAREZ-LÁINEZ M. Functionalization of polyacrylonitrile nanofibers with β-cyclodextrin for the capture of formaldehyde[J]. Materials & Design, 2016, 95: 632-640. |
| [33] |
NATH R K, ZAIN M F M, JAMIL M. An environment-friendly solution for indoor air purification by using renewable photocatalysts in concrete: A review[J]. Renewable and Sustainable Energy Reviews, 2016, 62: 1184-1194. DOI:10.1016/j.rser.2016.05.018 |
| [34] |
AL-GHOUTI M A, DA'ANA D A. Guidelines for the use and interpretation of adsorption isotherm models: A review[J]. Journal of Haza rdous Materials, 2020, 393: 122383. DOI:10.1016/j.jhazmat.2020.122383 |
| [35] |
ZHU L L, SHEN D K, LUO K H. A critical review on VOCs adsorption by different porous materials: Species, mechanisms and modification methods[J]. Journal of Hazardous Materials, 2020, 389: 122102. DOI:10.1016/j.jhazmat.2020.122102 |
| [36] |
FABBRIZZI L. Beyond the molecule: Intermolecular forces from gas liquefaction to X-H · · ·π hydrogen bonds[J]. ChemPlusChem, 2022, 87(1): e202100243. DOI:10.1002/cplu.202100243 |
| [37] |
HU P B, WENG Q Y, LI D L, et al. Research on the removal of As2O3 by γ-Al2O3 adsorption based on density functional theory[J]. Chemosphere, 2020, 257: 127243. DOI:10.1016/j.chemosphere.2020.127243 |
| [38] |
XU J X, ZHU F H, GE X L, et al. Research progress on volatile organic compounds emissions from coal-fired power plants[J]. Current Pollution Reports, 2022, 8(3): 303-314. DOI:10.1007/s40726-022-00225-8 |
| [39] |
SHI G B, HE S, CHEN G Y, et al. Crayfish shell-based micro-mesoporous activated carbon: Insight into preparation and gaseous benzene adsorption mechanism[J]. Chemical Engineering Journal, 2022, 428: 131148. DOI:10.1016/j.cej.2021.131148 |
| [40] |
JUNG C R, NISHIHAMA Y, NAKAYAMA S F, et al. Indoor air quality of 5, 000 households and its determinants. Part B: Volatile organic compounds and inorganic gaseous pollutants in the Japan Environment and Children's study[J]. Environmental Research, 2021, 197: 111135. DOI:10.1016/j.envres.2021.111135 |
| [41] |
KIM C, CHOI W, CHOI M. SO2-resistant amine-containing CO2 adsorbent with a surface protection layer[J]. ACS Applied Materials & Interfaces, 2019, 11(18): 16586-16593. |
| [42] |
NA C J, YOO M J, TSANG D C W, et al. High-performance materials for effective sorptive removal of formaldehyde in air[J]. Journal of Hazardous Materials, 2019, 366: 452-465. DOI:10.1016/j.jhazmat.2018.12.011 |
| [43] |
TAN L, WANG J G, CAI B H, et al. Nitrogen-rich layered carbon for adsorption of typical volatile organic compounds and low-temperature thermal regeneration[J]. Journal of Hazardous Materials, 2022, 424: 127348. DOI:10.1016/j.jhazmat.2021.127348 |
| [44] |
WANG X Z, KUKKAR D, YOUNIS S A, et al. The co-adsorption potential of metal-organic framework/activated carbon composites against both polar and non-polar volatile organic compounds in air[J]. Separation and Purification Technology, 2023, 306: 122594. DOI:10.1016/j.seppur.2022.122594 |
| [45] |
LI X Q, ZHANG L, YANG Z Q, et al. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: A review[J]. Separation and Purification Technology, 2020, 235: 116213. DOI:10.1016/j.seppur.2019.116213 |
| [46] |
ASIM N, BADIEI M, TORKASHVAND M, et al. Inorganic-based adsorbent materials for the removal of gaseous pollutants[J]. International Journal of Environmental Science and Technology, 2022, 19(6): 5731-5752. DOI:10.1007/s13762-021-03489-7 |
| [47] |
GUO X C, LI X Y, GAN G Q, et al. Functionalized activated carbon for competing adsorption of volatile organic compounds and water[J]. ACS Applied Materials & Interfaces, 2021, 13(47): 56510-56518. |
| [48] |
PRAHAS D, KARTIKA Y, INDRASWATI N, et al. Activated carbon from jackfruit peel waste by H3PO4 chemical activation: Pore structure and surface chemistry characterization[J]. Chemical Engineering Journal, 2008, 140(1/3): 32-42. |
| [49] |
CARDOSO B, MESTRE A S, CARVALHO A P, et al. Activated carbon derived from cork powder waste by KOH activation: Preparation, characterization, and VOCs adsorption[J]. Industrial & Engineering Chemistry Research, 2008, 47(16): 5841-5846. |
| [50] |
LI L, LIU S Q, LIU J X. Surface modification of coconut shell based activated carbon for the improvement of hydrophobic VOC removal[J]. Journal of Hazardous Materials, 2011, 192(2): 683-690. DOI:10.1016/j.jhazmat.2011.05.069 |
| [51] |
SILVESTRE-ALBERO A, SILVESTRE-ALBERO J, SEPÚLVEDA-ESCRIBANO A, et al. Ethanol removal using activated carbon: Effect of porous structure and surface chemistry[J]. Microporous and Mesoporous Materials, 2009, 120(1/2): 62-68. |
| [52] |
GIL R R, RUIZ B, LOZANO M S, et al. VOCs removal by adsorption onto activated carbons from biocollagenic wastes of vegetable tanning[J]. Chemical Engineering Journal, 2014, 245: 80-88. DOI:10.1016/j.cej.2014.02.012 |
| [53] |
ZHOU Y, ZHOU L, ZHANG X H, et al. Preparation of zeolitic imidazolate framework-8/graphene oxide composites with enhanced VOCs adsorption capacity[J]. Microporous and Mesoporous Materials, 2016, 225: 488-493. DOI:10.1016/j.micromeso.2016.01.047 |
| [54] |
YU W B, YUAN P, LIU D, et al. Facile preparation of hierarchically porous diatomite/MFI-type zeolite composites and their performance of benzene adsorption: The effects of NaOH etching pretreatment[J]. Journal of Hazardous Materials, 2015, 285: 173-181. DOI:10.1016/j.jhazmat.2014.11.034 |
| [55] |
BOYCHEVA S, ZGUREVA D, VÁCLAVÍKOVÁ M, et al. Studies on non-modified and copper-modified coal ash zeolites as heterogeneous catalysts for VOCs oxidation[J]. Journal of Hazardous Materials, 2019, 361: 374-382. DOI:10.1016/j.jhazmat.2018.07.020 |
| [56] |
BHATIA S, ABDULLAH A Z, WONG C T. Adsorption of butyl acetate in air over silver-loaded Y and ZSM-5 zeolites: Experimental and modelling studies[J]. Journal of Hazardous Materials, 2009, 163(1): 73-81. DOI:10.1016/j.jhazmat.2008.06.055 |
| [57] |
EDDAOUDI M, KIM J, ROSI N, et al. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage[J]. Science, 2002, 295(5554): 469-472. DOI:10.1126/science.1067208 |
| [58] |
JHUNG S H, LEE J H, YOON J W, et al. Microwave synthesis of chromium terephthalate MIL-101 and its benzene sorption ability[J]. Advanced Materials, 2007, 19(1): 121-124. DOI:10.1002/adma.200601604 |
| [59] |
LU S C, LIU Q L, HAN R, et al. Potential applications of porous organic polymers as adsorbent for the adsorption of volatile organic compounds[J]. Journal of Environmental Sciences, 2021, 105: 184-203. DOI:10.1016/j.jes.2021.01.007 |
| [60] |
HUANG X L, TANG M H, LI H X, et al. Adsorption of multicomponent VOCs on various biomass-derived hierarchical porous carbon: A study on adsorption mechanism and competitive effect[J]. Chemosphere, 2023, 313: 137513. DOI:10.1016/j.chemosphere.2022.137513 |
| [61] |
MA X W, YANG L J, WU H. Removal of volatile organic compounds from the coal-fired flue gas by adsorption on activated carbon[J]. Journal of Cleaner Production, 2021, 302: 126925. DOI:10.1016/j.jclepro.2021.126925 |
| [62] |
AÇIN OK R, KUTLUAY S. Designing novel perlite-Fe3O4@SiO2@8-HQ-5-SA as a promising magnetic nanoadsorbent for competitive adsorption of multicomponent VOCs[J]. Chemosphere, 2023, 338: 139636. DOI:10.1016/j.chemosphere.2023.139636 |
| [63] |
LI W L, JIANG Q G, LI D D, et al. Density functional theory investigation on selective adsorption of VOCs on borophene[J]. Chinese Chemical Letters, 2021, 32(9): 2803-2806. DOI:10.1016/j.cclet.2021.01.026 |
| [64] |
YANG F, LI W H, ZHONG X, et al. The alkaline sites integrated into biomass-carbon reinforce selective adsorption of acetic acid: In situ implanting MgO during activation operation[J]. Separation and Purification Technology, 2022, 297: 121415. DOI:10.1016/j.seppur.2022.121415 |
| [65] |
SEVERINO M I, AL MOHTAR A, SOARES C V, et al. MOFs with open metal(Ⅲ) sites for the environmental capture of polar volatile organic compounds[J]. Angewandte Chemie International Edition, 2023, 62(6): e202211583. DOI:10.1002/anie.202211583 |
| [66] |
HUANG X L, LI H X, WANG L, et al. Removal of toluene and SO2 by hierarchical porous carbons: A study on adsorption selectivity and mechanism[J]. Environmental Science and Pollution Research, 2022, 29(19): 29117-29129. DOI:10.1007/s11356-021-18380-8 |
| [67] |
SAKAI M, FUJIMAKI N, SASAKI Y, et al. Preferential adsorption of propylene over propane on a Ag-exchanged X-type zeolite membrane[J]. ACS Applied Materials & Interfaces, 2020, 12(21): 24086-24092. |
| [68] |
OU R, ZHU W J, LI L L, et al. Boosted capture of volatile organic compounds in adsorption capacity and selectivity by rationally exploiting defect-engineering of UiO-66(Zr)[J]. Separation and Purification Technology, 2021, 266: 118087. DOI:10.1016/j.seppur.2020.118087 |
| [69] |
KHARAROODI M G, HAGHIGHAT F, LEE C S. Develop and validate a mathematical model to estimate the removal of indoor VOCs by carbon filters[J]. Building and Environment, 2023, 233: 110082. DOI:10.1016/j.buildenv.2023.110082 |
| [70] |
SHEN X H, OU R, LU Y T, et al. Engineering adsorption case for efficient capture of VOCs using biomass-based corncobs via a carbonized strategy[J]. ChemistrySelect, 2020, 5(29): 9162-9169. DOI:10.1002/slct.202002086 |
| [71] |
SHEN X H, OU R, LU Y T, et al. Record-high capture of volatile benzene and toluene enabled by activator implant-optimized banana peel-derived engineering carbonaceous adsorbents[J]. Environment International, 2020, 143: 105774. DOI:10.1016/j.envint.2020.105774 |
| [72] |
YANG C T, MIAO G, PI Y H, et al. Abatement of various types of VOCs by adsorption/catalytic oxidation: A review[J]. Chemical Engineering Journal, 2019, 370: 1128-1153. DOI:10.1016/j.cej.2019.03.232 |
| [73] |
NOMURA A, JONES C W. Amine-functionalized porous silicas as adsorbents for aldehyde abatement[J]. ACS Applied Materials & Interfaces, 2013, 5(12): 5569-5577. |
| [74] |
LILLO-RÓDENAS M A, CAZORLA-AMORÓS D, LINARES-SOLANO A. Behaviour of activated carbons with different pore size distributions and surface oxygen groups for benzene and toluene adsorption at low concentrations[J]. Carbon, 2005, 43(8): 1758-1767. DOI:10.1016/j.carbon.2005.02.023 |
| [75] |
ZHU W J, SHEN X H, OU R, et al. Superhigh selective capture of volatile organic compounds exploiting cigarette butts-derived engineering carbonaceous adsorbent[J]. Chinese Journal of Chemical Engineering, 2022, 46: 194-206. DOI:10.1016/j.cjche.2021.01.012 |
| [76] |
MAO H Y, ZHOU D G, HASHISHO Z, et al. Microporous activated carbon from pinewood and wheat straw by microwave-assisted KOH treatment for the adsorption of toluene and acetone vapors[J]. RSC Advanc es, 2015, 5(45): 36051-36058. DOI:10.1039/C5RA01320H |
| [77] |
WANG X J, MA C, XIAO J, et al. Benzene/toluene/water vapor adsorption and selectivity of novel C-PDA adsorbents with high uptakes of benzene and toluene[J]. Chemical Engineering Journal, 2018, 335: 970-978. DOI:10.1016/j.cej.2017.10.102 |
| [78] |
MOHAN V, MARAPPAN G, KALIDOSS R, et al. Highly selective toluene adsorption studies of holey graphene oxide thin film using scanning Kelvin probe[J]. Materials Letters, 2023, 340: 134124. DOI:10.1016/j.matlet.2023.134124 |
| [79] |
SUMIDA R, MATSUMOTO T, YOKOI T, et al. A porous polyaromatic solid for vapor adsorption of xylene with high efficiency, selectivity, and reusability[J]. Chemistry-A European Journal, 2022, 28(72): e202202825. DOI:10.1002/chem.202202825 |
| [80] |
ROCHARD G, OLIVET L, TANNOUS M, et al. Recent advances in the catalytic treatment of volatile organic compounds: A review based on the mixture effect[J]. Catalysts, 2021, 11(10): 1218. DOI:10.3390/catal11101218 |
| [81] |
KAMAL M S, RAZZAK S A, HOSSAIN M M. Catalytic oxidation of volatile organic compounds (VOCs)-A review[J]. Atmospheric Environment, 2016, 140: 117-134. DOI:10.1016/j.atmosenv.2016.05.031 |
| [82] |
SHAYEGAN Z, LEE C S, HAGHIGHAT F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase-A review[J]. Chemical Engineering Journal, 2018, 334: 2408-2439. DOI:10.1016/j.cej.2017.09.153 |
| [83] |
WANG B, LIANG Y J, TONG K B, et al. What is the role of interface in the catalytic elimination of multi-carbon air pollutants?[J]. Chemosphere, 2023, 338: 139547. DOI:10.1016/j.chemosphere.2023.139547 |
| [84] |
CHEN X, CHEN X, CAI S C, et al. MnOx/Cr2O3 composites prepared by pyrolysis of Cr-MOF precursors containing in situ assembly of MnOx as high stable catalyst for toluene oxidation[J]. Applied Surface Science, 2019, 475: 312-324. DOI:10.1016/j.apsusc.2018.12.277 |
| [85] |
JAFARI A J, ARFAEINIA H, RAMAVANDI B, et al. Ozone-assisted photocatalytic degradation of gaseous toluene from waste air stream using silica-functionalized graphene oxide/ZnO coated on fiberglass: Performance, intermediates, and mechanistic pathways[J]. Air Quality, Atmosphere & Health, 2019, 12(10): 1181-1188. |
| [86] |
YUE X C, MA N L, SONNE C, et al. Mitigation of indoor air pollution: A review of recent advances in adsorption materials and catalytic oxidation[J]. J ournal of Hazard ous Mater ials, 2021, 405: 124138. DOI:10.1016/j.jhazmat.2020.124138 |
| [87] |
JAISON A, MOHAN A, LEE Y C. Recent developments in photocatalytic nanotechnology for purifying air polluted with volatile organic compounds: Effect of operating parameters and catalyst deactivation[J]. Catalysts, 2023, 13(2): 407. DOI:10.3390/catal13020407d.2016.07.005 |
| [88] |
HUANG H B, LIU G Y, ZHAN Y J, et al. Photocatalytic oxidation of gaseous benzene under VUV irradiation over TiO2/zeolites catalysts[J]. Catalysis Today, 2017, 281: 649-655. DOI:10.1016/j.cattod.2016.07.005 |
| [89] |
NIE L, DUAN B, LU A, et al. Pd/TiO2 @ carbon microspheres derived from chitin for highly efficient photocatalytic degradation of volatile organic compounds[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(1): 1658-1666. |
| [90] |
CHUN H H, JO W K. Heterogeneous decomposition of volatile organic compounds by visible-light activated N, C, S-embedded titania[J]. Journal of Nanoscience and Nanotechnology, 2016, 16(5): 4544-4553. DOI:10.1166/jnn.2016.10976 |
| [91] |
JAFARI A J, KALANTARY R R, ESRAFILI A, et al. Synthesis of silica-functionalized graphene oxide/ZnO coated on fiberglass and its application in photocatalytic removal of gaseous benzene[J]. Process Safety and Environmental Protection, 2018, 116: 377-387. DOI:10.1016/j.psep.2018.03.015 |
| [92] |
JI J, XU Y, HUANG H B, et al. Mesoporous TiO2 under VUV irradiation: Enhanced photocatalytic oxidation for VOCs degradation at room temperature[J]. Chemical Engineering Journal, 2017, 327: 490-499. DOI:10.1016/j.cej.2017.06.130 |
| [93] |
VAN DURME J, DEWULF J, SYSMANS W, et al. Efficient toluene abatement in indoor air by a plasma catalytic hybrid system[J]. Applied Catalysis B: Environmental, 2007, 74(1/2): 161-169. |
| [94] |
WEON S, KIM J, CHOI W. Dual-components modified TiO2 with Pt and fluoride as deactivation-resistant photocatalyst for the degradation of volatile organic compound[J]. Applied Catalysis B : Environmental, 2018, 220: 1-8. DOI:10.1016/j.apcatb.2017.08.036 |
| [95] |
ZHONG L X, BRANCHO J J, BATTERMAN S, et al. Experimental and modeling study of visible light responsive photocatalytic oxidation (PCO) materials for toluene degradation[J]. Applied Catalys is B : Environmental, 2017, 216: 122-132. DOI:10.1016/j.apcatb.2017.05.047 |
| [96] |
WEON S, CHOI E, KIM H, et al. Active {001} facet exposed TiO2 nanotubes photocatalyst filter for volatile organic compounds removal: From material development to commercial indoor air cleaner application[J]. Environmental Science & Technology, 2018, 52(16): 9330-9340. |
| [97] |
LI M, LU B, KE Q F, et al. Synergetic effect between adsorption and photodegradation on nanostructured TiO2/activated carbon fiber felt porous composites for toluene removal[J]. Journal of Hazardous Materials, 2017, 333: 88-98. DOI:10.1016/j.jhazmat.2017.03.019 |
| [98] |
HAGHIGHATMAMAGHANI A, HAGHIGHAT F, LEE C S. Performance of various commercial TiO2 in photocatalytic degradation of a mixture of indoor air pollutants: Effect of photocatalyst and operating parameters[J]. S cience and Technology for the Built Environment, 2019, 25(5): 600-614. DOI:10.1080/23744731.2018.1556051 |
| [99] |
SIDHESWARAN M, TAVLARIDES L L. Visible light photocatalytic oxidation of toluene using a cerium-doped titania catalyst[J]. Industrial & Engineering Chemistry Research, 2008, 47(10): 3346-3357. |
| [100] |
XIE R J, LEI D X, ZHAN Y J, et al. Efficient photocatalytic oxidation of gaseous toluene over F-doped TiO2 in a wet scrubbing process[J]. Chemical Engineering Journal, 2020, 386: 121025. DOI:10.1016/j.cej.2019.02.112 |
| [101] |
WEON S, CHOI J, PARK T, et al. Freestanding doubly open-ended TiO2 nanotubes for efficient photocatalytic degradation of volatile organic compounds[J]. Applied Catalysis B : Environmental, 2017, 205: 386-392. DOI:10.1016/j.apcatb.2016.12.048 |
| [102] |
WANG S J, ZHANG X Y, SU D, et al. Electrospinning Ag-TiO2 nanorod-loaded air treatment filters and their applications in air purification[J]. Molecules, 2020, 25(15): 3369. DOI:10.3390/molecules25153369 |
| [103] |
LIANG W J, LI J, JIN Y Q. Photocatalytic degradation of gaseous acetone, toluene, and p-xylene using a TiO2 thin film[J]. Journal of Environmental Science and Health, Part A : Toxic/Hazardous Substances & Environmental Engineering, 2010, 45(11): 1384-1390. |
| [104] |
DONG F, GUO S, WANG H Q, et al. Enhancement of the visible light photocatalytic activity of C-doped TiO2 nanomaterials prepared by a green synthetic approach[J]. The Journal of Physical Chemistry C, 2011, 115(27): 13285-13292. DOI:10.1021/jp111916q |
| [105] |
DONG X A, CUI Z H, SUN Y J, et al. Humidity-independent photocatalytic toluene mineralization benefits from the utilization of edge hydroxyls in layered double hydroxides (LDHs): A combined operando and theoretical investigation[J]. ACS Catalysis, 2021, 11(13): 8132-8139. DOI:10.1021/acscatal.1c01599 |
| [106] |
LEI B, CUI W, CHEN P, et al. Rational design of LDH/Zn2SnO4 heterostructures for efficient mineralization of toluene through boosted interfacial charge separation[J]. Energy & Environmental Materials, 2023, 6(1): e12291. |
| [107] |
LIMMONGKON Y, JOHNS J, CHARERNTANYARAK L. Preparation of a TiO2-coated photocatalytic air filter for use with an electrostatic air filter pack for xylene removal[J]. ScienceAsia, 2013, 39(3): 284-293. DOI:10.2306/scienceasia1513-1874.2013.39.284 |
| [108] |
ZHANG J J, VIKRANT K, KIM K H, et al. Unveiling the collective effects of moisture and oxygen on the photocatalytic degradation of m-Xylene using a titanium dioxide supported platinum catalyst[J]. Chemical Engineering Journal, 2022, 439: 135747. DOI:10.1016/j.cej.2022.135747 |
| [109] |
PARVARI R, GHORBANI-SHAHNA F, BAHRAMI A, et al. A novel core-shell structured α-Fe2O3/Cu/g-C3N4 nanocomposite for continuous photocatalytic removal of air ethylbenzene under visible light irradiation[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2020, 399: 112643. DOI:10.1016/j.jphotochem.2020.112643 |
| [110] |
KAMAEI M, RASHEDI H, DASTGHEIB S M M, et al. Comparing photocatalytic degradation of gaseous ethylbenzene using N-doped and pure TiO2 nano-catalysts coated on glass beads under both UV and visible light irradiation[J]. Catalysts, 2018, 8(10): 466. DOI:10.3390/catal8100466 |
| [111] |
LI Q, ZHANG S W, XIA W J, et al. Surface design of g-C3N4 quantum dot-decorated TiO2(001) to enhance the photodegradation of indoor formaldehyde by experimental and theoretical investigation[J]. Ecotoxicology and Environmental Safety, 2022, 234: 113411. DOI:10.1016/j.ecoenv.2022.113411 |
| [112] |
VON BOEHN B, IMBIHL R. Dynamics of ultrathin vanadium oxide layers on Rh(111) and Rh(110) surfaces during catalytic reactions[J]. Frontiers in Chemis try, 2020, 8: 707. DOI:10.3389/fchem.2020.00707 |
| [113] |
AHMAD A, ALI M, AL-SEHEMI A G, et al. Carbon-integrated semiconductor photocatalysts for removal of volatile organic compounds in indoor environments[J]. Chemical Engineering Journal, 2023, 452: 139436. DOI:10.1016/j.cej.2022.139436 |
| [114] |
SUÁREZ S, JANSSON I, OHTANI B, et al. From titania nanoparticles to decahedral anatase particles: Photocatalytic activity of TiO2/zeolite hybrids for VOCs oxidation[J]. Catalysis Today, 2019, 326: 2-7. DOI:10.1016/j.cattod.2018.09.004 |
| [115] |
HUANG Q Q, HU Y, PEI Y, et al. In situ synthesis of TiO2@NH2-MIL-125 composites for use in combined adsorption and photocatalytic degradation of formaldehyde[J]. Applied Catalysis B: Environmental, 2019, 259: 118106. DOI:10.1016/j.apcatb.2019.118106 |
| [116] |
JIANG K, ZHANG J, WAN Y F, et al. Effect of amination of titanium dioxide in the TiO2/rGO composite on the efficient photocatalytic removal of gaseous formaldehyde at room temperature[J]. Optical Materials, 2021, 114: 110913. DOI:10.1016/j.optmat.2021.110913 |
| [117] |
ZONG Z F, CHEN D P, ZHAO C X, et al. Photocatalytic degradation performance of gaseous formaldehyde by Ce-Eu/TiO2 hollow microspheres: From experimental evaluation to simulation[J]. Environmental Science and Pollution Research, 2021, 28(26): 34762-34775. DOI:10.1007/s11356-021-13112-4 |
| [118] |
YU L, WANG L, SUN X B, et al. Enhanced photocatalytic activity of rGO/TiO2 for the decomposition of formaldehyde under visible light irradiation[J]. Journal of Environmental Sciences, 2018, 73: 138-146. DOI:10.1016/j.jes.2018.01.022 |
| [119] |
ZHANG J, XU X, YANG H, et al. The preparation and characterization of TiO2/r-GO/Ag nanocomposites and its photocatalytic activity in formaldehyde degradation[J]. Environmental Technology, 2021, 42(2): 193-205. DOI:10.1080/09593330.2019.1625955 |
| [120] |
KONG L R, LI X X, SONG P, et al. Porous graphitic carbon nitride nanosheets for photocatalytic degradation of formaldehyde gas[J]. Chemical Physics Letters, 2021, 762: 138132. DOI:10.1016/j.cplett.2020.138132 |
| [121] |
SONG S Q, LU C H, WU X, et al. Strong base g-C3N4 with perfect structure for photocatalytically eliminating formaldehyde under visible-light irradiation[J]. Applied Catalysis B: Environmental, 2018, 227: 145-152. DOI:10.1016/j.apcatb.2018.01.014 |
| [122] |
HU C, TSAI W F, WEI W H, et al. Hydroxylation and sodium intercalation on g-C3N4 for photocatalytic removal of gaseous formaldehyde[J]. Carbon, 2021, 175: 467-477. DOI:10.1016/j.carbon.2021.01.112 |
| [123] |
LI X, QIAN X R, AN X H, et al. Preparation of a novel composite comprising biochar skeleton and "chrysanthemum" g-C3N4 for enhanced visible light photocatalytic degradation of formaldehyde[J]. Applied Surface Science, 2019, 487: 1262-1270. DOI:10.1016/j.apsusc.2019.05.195 |
| [124] |
LIU S H, LIN W X. A simple method to prepare g-C3N4-TiO2/waste zeolites as visible-light-responsive photocatalytic coatings for degradation of indoor formaldehyde[J]. Journal of Hazardous Materials, 2019, 368: 468-476. DOI:10.1016/j.jhazmat.2019.01.082 |
| [125] |
LI X, FANG G G, QIAN X R, et al. Z-scheme heterojunction of low conduction band potential MnO2 and biochar-based g-C3N4 for efficient formaldehyde degradation[J]. Chemical Engineering Journal, 2022, 428: 131052. DOI:10.1016/j.cej.2021.131052 |
| [126] |
SONG M T, WU Y H, DU C F, et al. S-scheme bismuth vanadate and carbon nitride integrating with dual-functional bismuth nanoparticles toward co-efficiently removal formaldehyde under full spectrum light[J]. Journal of Colloid and Interface Science, 2021, 588: 357-368. DOI:10.1016/j.jcis.2020.12.087 |
| [127] |
PENG S, YANG X X, STRONG J, et al. MnO2-decorated N-doped carbon nanotube with boosted activity for low-temperature oxidation of formaldehyde[J]. Journal of Hazardous Materials, 2020, 396: 122750. DOI:10.1016/j.jhazmat.2020.122750 |
| [128] |
LIU Y Q, ZHU C J, SUN J W, et al. In situ assembly of CQDs/Bi2WO6 for highly efficient photocatalytic degradation of VOCs under visible light[J]. New Journal of Chemistry, 2020, 44(8): 3455-3462. DOI:10.1039/C9NJ04957F |
| [129] |
DAI L, LI X Y, ZHANG L, et al. Facile preparation of FL-Ti3C2/BiOCl/SnO2 ternary composite for photocatalytic degradation of indoor formaldehyde[J]. Advanced Composites and Hybrid Materials, 2022, 5(3): 2285-2296. DOI:10.1007/s42114-021-00398-8 |
| [130] |
WANG T Q, WANG Y F, SUN M Z, et al. Thermally treated zeolitic imidazolate framework-8 (ZIF-8) for visible light photocatalytic degradation of gaseous formaldehyde[J]. Chemical Science, 2020, 11(26): 6670-6681. DOI:10.1039/D0SC01397H |
| [131] |
周羿彤, 王文苑, 顾益凡, 等. 室内空气净化器流动强化及效能提升方法[J]. 节能技术, 2020, 38(2): 151-155. ZHOU Y T, WANG W Y, GU Y F, et al. Methods of flow enhancement and performance improvement for indoor air cleaner[J]. Energy Conservation Technology, 2020, 38(2): 151-155. |
| [132] |
肖康, 王琼. 吸附法净化室内甲醛研究进展[J]. 化工进展, 2021, 40(10): 5747-5771. XIAO K, WANG Q. Progress in research on adsorption for abatement of indoor formaldehyde[J]. Chemical Industry and Engineering Progress, 2021, 40(10): 5747-5771. |
| [133] |
HU S C, CHEN Y C, LIN X Z, et al. Characterization and adsorption capacity of potassium permanganate used to modify activated carbon filter media for indoor formaldehyde removal[J]. Environmental Science and Pollution Research, 2018, 25(28): 28525-28545. DOI:10.1007/s11356-018-2681-z |
| [134] |
WANG D, YIN T T, ZHANG Y H, et al. Titania coated silica core-shell spheres with dual grain size as efficient photocatalysts[J]. Microporous and Mesoporous Materials, 2022, 338: 111966. DOI:10.1016/j.micromeso.2022.111966 |
| [135] |
石芳芳, 邱利民, 于川, 等. 室内空气净化技术及产品综述[J]. 制冷学报, 2014, 35(5): 14-18, 37. SHI F F, QIU L M, YU C, et al. Techniques and products for air purification[J]. Journal of Refrigeration, 2014, 35(5): 14-18, 37. |
| [136] |
康瑞鑫, 刘国华. 家用空气净化器捕获颗粒物原理与技术[J]. 建筑热能通风空调, 2018, 37(2): 76-80. KANG R X, LIU G H. Study of domestic air cleaners for particulate purification[J]. Building Energy & Environment, 2018, 37(2): 76-80. |
| [137] |
曾曜. 光催化技术在室内空气净化中的应用[J]. 绿色环保建材, 2017(3): 31. ZENG Y. Application of photocatalysis technology in indoor air purification[J]. Green Environmental Protection Building Materials, 2017(3): 31. |
| [138] |
LIU Q Y, WANG H D, TANG R, et al. Rutile TiO2 nanoparticles with oxygen vacancy for photocatalytic nitrogen fixation[J]. ACS Applied Nano Materials, 2021, 4(9): 8674-8679. DOI:10.1021/acsanm.1c02184 |
| [139] |
FAVAS P J C, PRATAS J, VARUN M, et al. Accumulation of uranium by aquatic plants in field conditions: Prospects for phytoremediation[J]. Science of the Total Environment, 2014, 470-471: 993-1002. DOI:10.1016/j.scitotenv.2013.10.067 |
| [140] |
WANG D Y, LUO H, LIU L X, et al. Adsorption characteristics and degradation mechanism of metronidazole on the surface of photocatalyst TiO2: A theoretical study[J]. Applied Surface Science, 2019, 478: 896-905. DOI:10.1016/j.apsusc.2019.02.052 |
| [141] |
SAFRI A, FLETCHER A J. Effective carbon/TiO2 gel for enhanced adsorption and demonstrable visible light driven photocatalytic performance[J]. Gels, 2022, 8(4): 215. DOI:10.3390/gels8040215 |
| [142] |
TRZECIAK M, MI DLICKI P, TRYBA B. Enhanced degradation of ethylene in thermo-photocatalytic process using TiO2/Nickel foam[J]. Materials, 2024, 17(1): 267. DOI:10.3390/ma17010267 |
| [143] |
LI Z Y, MAO Y, HUANG Y F, et al. Theoretical and experimental studies of highly efficient all-solid Z-scheme TiO2-TiC/g-C3N4 for photocatalytic CO2 reduction via dry reforming of methane[J]. Catalysis Science & Technology, 2022, 12(9): 2804-2818. |
| [144] |
HE K, ZHAO C, ZHAO G L, et al. Effects of pore size on the photocatalytic activity of mesoporous TiO2 prepared by a sol-gel process[J]. Journal of Sol-Gel Science and Technology, 2015, 75(3): 557-563. DOI:10.1007/s10971-015-3726-0 |
| [145] |
ZHANG H, WANG X, DING H. Clean, low-cost, and efficient nano-TiO2 composite photocatalyst: Preparation and performance in TiOSO4 hydrolysis system[J]. Chemical Physics Letters, 2023, 829: 140757. DOI:10.1016/j.cplett.2023.140757 |
| [146] |
XIAO C J, YAN Y K, WEN G D, et al. Regulating crystallinity in cellulose substrate to construct highly and homogeneously dispersed TiO2 for tetracycline hydrochloride adsorption[J]. Materials & Design, 2023, 226: 111620. |
| [147] |
DENG C, HONG R J, JING M, et al. Photocatalytic performance of TiO2 thin film decorated with Cu2O nanoparticles by laser ablation[J]. Optical Materials, 2019, 94: 130-137. DOI:10.1016/j.optmat.2019.05.030 |
| [148] |
FARZANEH A, JAVIDANI M, ESRAFILI M D, et al. Optical and photocatalytic characteristics of Al and Cu doped TiO2: Experimental assessments and DFT calculations[J]. Journal of Physics and Chemistry of Solids, 2022, 161: 110404. DOI:10.1016/j.jpcs.2021.110404 |
| [149] |
XU W, TANG H L, ZHOU N, et al. Enhanced photocatalytic activity of TiO2/Ag2O heterostructures by optimizing the separation of electric charges[J]. Vacuum, 2021, 190: 110283. DOI:10.1016/j.vacuum.2021.110283 |
| [150] |
SHEN Q Q, WANG Y, XUE J B, et al. The dual effects of RGO films in TiO2/CdSe heterojunction: Enhancing photocatalytic activity and improving photocorrosion resistance[J]. Applied Surface Science, 2019, 481: 1515-1523. DOI:10.1016/j.apsusc.2019.03.136 |
| [151] |
LI Y Y, SUN J, CHEN Y H, et al. Fabrication of an in situ-grown TiO2 nanowire thin film and its enhanced photocatalytic activity[J]. Environmental Science and Pollution Research, 2023, 30(34): 82560-82574. DOI:10.1007/s11356-023-28229-x |
| [152] |
LIN Z F, TONG X, SHEN W H, et al. Humidity impact on photo-catalytic degradation: Adsorption behavior simulations and catalytic reaction mechanisms for main gaseous pollutants in papermaking industry[J]. Journal of Cleaner Production, 2020, 244: 118863. DOI:10.1016/j.jclepro.2019.118863 |
| [153] |
LEE B X Y, HADIBARATA T, YUNIARTO A. Phytoremediation mechanisms in air pollution control: A review[J]. Water, Air, & Soil Pollution, 2020, 231(8): 437. |
| [154] |
MORIKAWA H, ERKIN Ö C. Basic processes in phytoremediation and some applications to air pollution control[J]. Chemosphere, 2003, 52(9): 1553-1558. DOI:10.1016/S0045-6535(03)00495-8 |
| [155] |
VISCONTI D, ÁLVAREZ-ROBLES M J, FIORENTINO N, et al. Use of Brassica juncea and Dactylis glomerata for the phytostabilization of mine soils amended with compost or biochar[J]. Chemosphere, 2020, 260: 127661. DOI:10.1016/j.chemosphere.2020.127661 |
| [156] |
LIU W S, CHEN Y Y, HUOT H, et al. Phytoextraction of rare earth elements from ion-adsorption mine tailings by Phytolacca americana: Effects of organic material and biochar amendment[J]. Journal of Cleaner Production, 2020, 275: 122959. DOI:10.1016/j.jclepro.2020.122959 |
| [157] |
WEYENS N, THIJS S, POPEK R, et al. The role of plant-microbe interactions and their exploitation for phytoremediation of air pollutants[J]. International Journal of Molecular Science, 2015, 16(10): 25576-25604. DOI:10.3390/ijms161025576 |
| [158] |
MOHSIN M, KUITTINEN S, SALAM M M A, et al. Chelate-assisted phytoextraction: Growth and ecophysiological responses by Salix schwerinii E.L Wolf grown in artificially polluted soils[J]. Journal of Geochemical Exploration, 2019, 205: 106335. DOI:10.1016/j.gexplo.2019.106335 |
| [159] |
GUARINO F, MIRANDA A, CASTIGLIONE S, et al. Arsenic phytovolatilization and epigenetic modifications in Arundo donax L. assisted by a PGPR consortium[J]. Chemosphere, 2020, 251: 126310. DOI:10.1016/j.chemosphere.2020.126310 |
| [160] |
SINGH V, PANDEY B, SUTHAR S. Phytotoxicity and degradation of antibiotic ofloxacin in duckweed (Spirodela polyrhiza) system[J]. Ecotoxicology and Environmental Safety, 2019, 179: 88-95. DOI:10.1016/j.ecoenv.2019.04.018 |
| [161] |
KAGALKAR A N, JADHAV M U, BAPAT V A, et al. Phytodegradation of the triphenylmethane dye Malachite Green mediated by cell suspension cultures of Blumea malcolmii Hook[J]. Bioresource Technology, 2011, 102(22): 10312-10318. DOI:10.1016/j.biortech.2011.08.101 |
| [162] |
KRISTANTI R A, KANBE M, HADIBARATA T, et al. Isolation and characterization of 3-nitrophenol-degrading bacteria associated with rhizosphere of Spirodela polyrrhiza[J]. Environmental Science and Pollution Research, 2012, 19(5): 1852-1858. DOI:10.1007/s11356-012-0836-x |
| [163] |
NAJEEB U, AHMAD W, ZIA M H, et al. Enhancing the lead phytostabilization in wetland plant Juncus effusus L. through somaclonal manipulation and EDTA enrichment[J]. Arabian Journal of Chemistry, 2017, 10(Suppl.2): S3310-S3317. |
| [164] |
TAPIA Y, BUSTOS P, SALAZAR O, et al. Phytostabilization of Cu in mine tailings using native plant Carpobrotus aequilaterus and the addition of potassium humates[J]. Journal of Geochemical Exploration, 2017, 183: 102-113. DOI:10.1016/j.gexplo.2017.10.008 |
| [165] |
LI Y F, ZHANG J F, ZHU G B, et al. Phytoextraction, phytotransformation and rhizodegradation of ibuprofen associated with Typha angustifolia in a horizontal subsurface flow constructed wetland[J]. Water Research, 2016, 102: 294-304. DOI:10.1016/j.watres.2016.06.049 |
| [166] |
DOMINGUEZ J J A, INOUE C, CHIEN M F. Hydroponic approach to assess rhizodegradation by sudangrass (Sorghum x drummondii) reveals pH-and plant age-dependent variability in bacterial degradation of polycyclic aromatic hydrocarbons (PAHs)[J]. Journal of Hazardous Materials, 2020, 387: 121695. DOI:10.1016/j.jhazmat.2019.121695 |
| [167] |
LEE M, YANG M. Rhizofiltration using sunflower (Helianthus annuus L.) and bean (Phaseolus vulgaris L. var. vulgaris) to remediate uranium contaminated groundwater[J]. Journal of Hazardous Materials, 2010, 173(1/3): 589-596. |
| [168] |
PENG W X, YUE X C, CHEN H L, et al. A review of plants formaldehyde metabolism: Implications for hazardous emissions and phytoremediation[J]. Journal of Hazardous Materials, 2022, 436: 129304. DOI:10.1016/j.jhazmat.2022.129304 |
| [169] |
DINGLE P, FRANKLIN P. Formaldehyde levels and the factors affecting these levels in homes in Perth, western Australia[J]. Indoor and Built Environment, 2002, 11(2): 111-116. DOI:10.1177/1420326X0201100206 |
| [170] |
LIU Y, YUAN Y, LEI X Y, et al. Purification and characterisation of two enzymes related to endogenous formaldehyde in Lentinula edodes[J]. Food Chem istry, 2013, 138(4): 2174-2179. DOI:10.1016/j.foodchem.2012.12.038 |
| [171] |
ROTTENBERGER S, KUHN U, WOLF A, et al. Formaldehyde and acetaldehyde exchange during leaf development of the Amazonian deciduous tree species Hymenaea courbaril[J]. Atmospheric Environment, 2005, 39(12): 2275-2279. DOI:10.1016/j.atmosenv.2004.12.027 |
| [172] |
WANG L P, SHENG Q Q, ZHANG Y L, et al. Tolerance of fifteen hydroponic ornamental plant species to formaldehyde stress[J]. Environmental Pollution, 2020, 265: 115003. DOI:10.1016/j.envpol.2020.115003 |
| [173] |
陶雪琴, 卢桂宁, 周康群, 等. 大气化学污染的植物净化研究进展[J]. 生态环境, 2007, 16(5): 1546-1550. TAO X Q, LU G N, ZHOU K Q, et al. Phytodecontamination of atmosphere chemical pollution: A review[J]. Ecology and Environment, 2007, 16(5): 1546-1550. DOI:10.3969/j.issn.1674-5906.2007.05.042 |
| [174] |
WANG R, ZENG Z D, GUO H X, et al. Over-expression of the Arabidopsis formate dehydrogenase in chloroplasts enhances formaldehyde uptake and metabolism in transgenic tobacco leaves[J]. Planta, 2018, 247(2): 339-354. DOI:10.1007/s00425-017-2790-9 |
| [175] |
SU Y H, LIANG H X, ZHAO S Y, et al. Removal efficiency and mechanisms of formaldehyde by five species of plants in air-plant-water system[J]. Human and Ecological Risk Assessment: An International Journal, 2019, 25(4): 1059-1071. DOI:10.1080/10807039.2018.1474432 |
| [176] |
MARCHAND C, LE CALVÉ S, MIRABEL P, et al. Concentrations and determinants of gaseous aldehydes in 162 homes in Strasbourg (France)[J]. Atmospher ic Environment, 2008, 42(3): 505-516. DOI:10.1016/j.atmosenv.2007.09.054 |
| [177] |
HOPKINSON R J, LEUNG I K H, SMART T J, et al. Studies on the glutathione-dependent formaldehyde-activating enzyme from Paracoccus denitrificans[J]. PLoS One, 2015, 10(12): e0145085. DOI:10.1371/journal.pone.0145085 |
| [178] |
LEE J H. An overview of phytoremediation as a potentially promising technology for environmental pollution control[J]. Biotechnology and Bioprocess Engineering, 2013, 18(3): 431-439. DOI:10.1007/s12257-013-0193-8 |
| [179] |
SECO R, PEÑUELAS J, FILELLA I. Formaldehyde emission and uptake by Mediterranean trees Quercus ilex and Pinus halepensis[J]. Atmospheric Environment, 2008, 42(34): 7907-7914. DOI:10.1016/j.atmosenv.2008.07.006 |
| [180] |
SCHMITZ H, HILGERS U, WEIDNER M. Assimilation and metabolism of formaldehyde by leaves appear unlikely to be of value for indoor air purification[J]. New Phytologist, 2000, 147(2): 307-315. DOI:10.1046/j.1469-8137.2000.00701.x |
| [181] |
HASLAM R, RUST S, PALLETT K, et al. Cloning and characterisation of S-formylglutathione hydrolase from Arabidopsis thaliana: A pathway for formaldehyde detoxification[J]. Plant Physiology and Biochemistry, 2002, 40(4): 281-288. DOI:10.1016/S0981-9428(02)01378-5 |
| [182] |
XU Z J, QIN N, WANG J G, et al. Formaldehyde biofiltration as affected by spider plant[J]. Bioresource Technology, 2010, 101(18): 6930-6934. DOI:10.1016/j.biortech.2010.03.128 |