能源和环境问题相互依存、日益严峻,并受到全球的关注,开发太阳能、风能等清洁能源以减少对化石能源的依赖是解决问题的关键之一。氢作为一种清洁无污染的绿色能源及能源载体,其开发及利用受到了广泛关注[1~3]。通过低成本、无污染的光催化分解水技术,将太阳能转化为绿色、可储存的氢能,是一种从根本上解决能源危机和环境污染的理想途径[4]。图 1展示了光催化分解水产氢系统及氢能利用示意图。目前,国内外已有很多光催化制氢方面的综述,如对某特定光催化材料的研究进展[5~9]、对光催化产氢材料及产氢体系的综合评述[10~15]以及光催化反应器的研究进展[16, 17]等。本综述围绕目前光催化分解水催化剂的研究进展,对催化剂的修饰与改性技术进行了总结和评述。
|
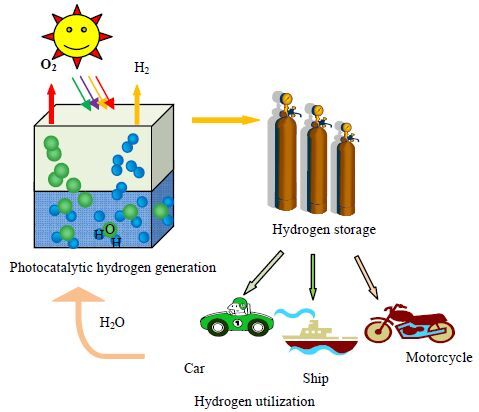
图 1 光催化分解水产氢及氢能利用示意图 Fig.1 Schematic diagram of photocatalytic hydrogen evolution in the hydrogen energy system |
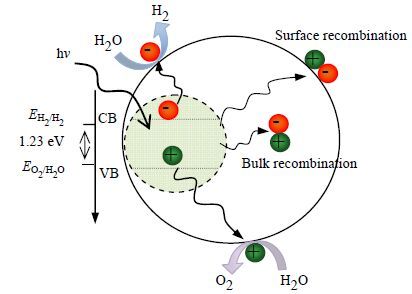
在光催化过程中,催化剂的电子结构起到了关键作用。半导体的导带及价带之间的能级差称为禁带宽度。当入射光量子能量等于或者大于半导体的禁带宽度时,价带电子激发跃迁至导带,相应的在价带上产生空穴。光生电子与空穴分离并迁移到催化剂表面,其中电子与水进行还原反应产生氢气,空穴与水或其他空穴捕集剂进行氧化反应,从而实现直接或间接的分解水过程。同时,光生电子和空穴会迅速的在催化剂内部及表面发生复合,从而影响催化剂表面的氧化与还原反应,降低光催化效率。因此,在光催化过程中,加速电子-空穴对的分离,降低电子空穴复合速率,对提高光催化效率至关重要。图 2为半导体光催化分解水产氢机理图(根据参考文献[18]修改)。
|
图 2 半导体光催化分解水产氢机理图(根据参考文献[18]修改) Fig.2 Processes in photocatalytic water splitting (Modified from ref[18]) |
在光催化分解水体系中,产氢效率受多种因素的影响制约。简单地说,主要包括催化剂种类、半导体材料的能带结构、催化剂晶粒大小及形貌、光催化反应条件等。同时,逆反应及催化剂的光腐蚀等也会影响产氢效率。
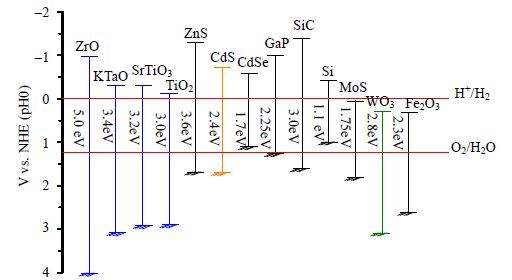
(1) 能带结构。半导体的能带结构是限制其产氢能力的最根本因素,价带导带位置直接决定了催化剂是否具备分解水的能力。由于水是一种稳定的化合物,分解水产氢产氧在热力学上是一个非自发过程,需要标准吉布斯自由能增加237 kJ⋅mol-1 (如式1) 。为了在热力学上满足整体分解水条件,半导体催化剂的价带(VB)位置必须在氧的电极电位(EO2/H2O = 1.23 V vs NHE,pH = 0) 之下(> 1.23 V),导带(CB)位置必须在氢的电极电位(EO2/H2O = 0 V vs NHE,pH = 0) 之上(< 0 V),即催化剂的能带结构至少需要大于1.23 eV[12, 19]。一般来说,催化剂导带位置越负,导带电子所具备的还原能力越强,价带位置越正,价带空穴所具备的氧化能力越强。然而,催化剂的能带结构和位置对不同波段光的利用率也有影响,从而影响着光催化产氢效率。禁带宽度较宽的催化剂(> 3.2 eV)只能利用紫外光(λ < 400 nm),如:TiO2[20]、ZrO2[21]、SrTiO3[22]、Ga2O3[23]、CeO2[24]、ZnS[25]、GaN[26]等,为了更充分地利用太阳光,应尽量开发可见光响应催化剂。图 3展示了一些催化剂的能带结构与水分解氧化还原电势的关系图,理论上,多种催化剂满足同时产氢产氧条件,WO3、MoS2、Fe2O3等催化剂的导带位置低于氢的电极电位而不能实现光催化产氢,ZrO2、SrTiO3、KTaO3等催化剂的禁带宽度较大而无法利用可见光[27]。
| ${{\text{H}}_{\text{2}}}\text{O}\to \frac{\text{1}}{\text{2}}{{\text{O}}_{\text{2}}}+{{\text{H}}_{\text{2}}}\text{; }\!\!\Delta\!\!\text{ }G=+\text{237 kJ}\cdot mo{{l}^{-1}}$ | (1) |
|
图 3 催化剂能带结构与水分解氧化还原电势关系图[27] Fig.3 Relationship between band structure of semiconductors and redox potentials of water splitting[27] |
(2) 催化剂的其它物理化学性能。光催化分解水产氢过程中,催化剂的晶体结构、结晶度、颗粒大小、形貌等物理化学性能很大程度上影响着催化剂的光催化性能。以TiO2为例,具有亚稳态晶型的锐钛矿相TiO2较具有稳定晶相的金红石相TiO2往往表现出更优异的光催化性能[13]。同时,催化剂的结晶度越好,晶体内部缺陷越少,晶体内部电子空穴复合(bulk recombination)越少,光催化效果越好。催化剂颗粒越小,电子-空穴对迁移到表面的距离越短,也可能减少晶体内部电子-空穴对的复合[12]。催化剂的晶粒大小、形貌、孔径分布等因素共同决定了催化剂的比表面积及表面活性位点的多少,进而影响整个催化反应过程。一般来说,晶粒尺寸较小、分散较均匀的催化剂拥有较大的比表面积,从而更有利于光催化反应。通过采用不同的制备方法及制备条件,可以制备出不同结晶度及形貌的催化剂,如制备纳米片状、多孔状及具有不同晶面暴露的催化剂等[28~32],改善光催化性能。
(3) 光催化反应条件。在光催化分解水过程中,体系中牺牲剂的选取、环境温度、pH值、过电势等反应条件均会对产氢效率产生一定的影响。由于实现整体分解水是困难的,通过加入牺牲剂(电子给体或电子受体)实现间接分解水成为研究的主流,即产氢或产氧“半反应”。在产氢“半反应”中,加入的牺牲剂能消耗空穴,从而阻止光生电子与空穴的复合,加速产氢速率。常见的空穴捕集剂有甲醇、乙醇、腐殖酸、甘油(丙三醇)等[33, 34]。另外,对于CdS、ZnS等硫族催化剂,在光催化反应过程中本身存在光腐蚀问题(式2) ,常需添加S2-/SO32-[35, 36]等作为牺牲剂,减缓催化剂的光腐蚀,从而增强体系的析氢能力。通过改变光催化体系中的温度条件,改变催化剂表面氢气的脱附能力,同样可以改变催化体系的析氢能力[13]。
| $CdS+2{{h}^{+}}\to C{{d}^{2+}}+S$ | (2) |
可用于光催化分解水的催化剂很多,但多数催化剂缺乏合适的能带结构,且光生电子-空穴对易复合,从而导致光催化产氢效率,尤其是可见光产氢效率不理想。为了实现催化剂的可见光化、提高光催化产氢效率,通常需要对催化剂进行修饰与改性,有效调变催化剂的能带结构、降低电子-空穴对的复合速率、提高催化剂的稳定性。
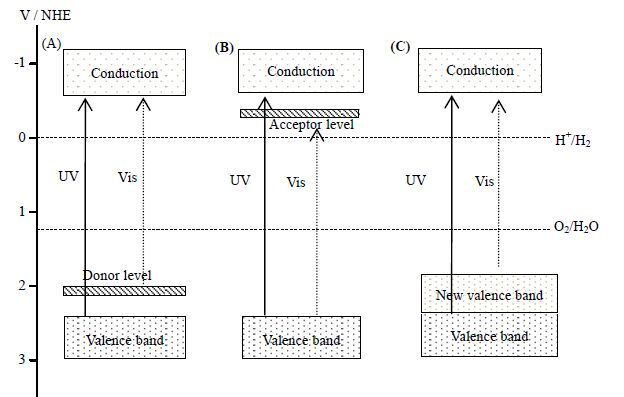
3.1 催化剂掺杂改性催化剂掺杂改性的主要目的是在催化剂中引入杂质能级,缩短禁带宽度,从而实现催化剂的可见光激发,主要包括金属离子掺杂和非金属离子掺杂。金属离子掺杂(图 4(A)、(B))通过引入金属离子在本征半导体中形成杂质能级,在催化剂VB上方形成施主能级(donor level)或在CB下方形成受主能级(acceptor level),从而使能量较小的光子也能激发掺杂能级,扩展光谱响应范围。目前针对金属离子掺杂已有大量的研究[37~39],常见的掺杂金属有Fe、Cu、Cr、Ni、Mn、Sn、V等[40~43]。非金属离子掺杂(图 4 C)主要以C[44]、N[45]、S[46]等小原子半径元素为主,利用掺杂元素外层S、P轨道与本征导带和价带重迭使原催化剂VB上移,从而缩小催化剂的禁带宽度,扩展光谱响应范围。也有人认为,并不是VB位置上移使得非金属离子掺杂的催化剂光吸收边红移,而是非金属离子掺杂后在基底催化剂的禁带内形成局域能级或引进氧空位造成的[47]。大量计算和实验结果表明,离子掺杂能有效扩大光响应范围、提升可见光下分解水速率,是一种调变半导体禁带宽度、调节价带导带位置的有效途径[48~50]。Sun等[51]研究了Ce/N共掺杂TiO2催化剂,发现Ce、N、Ce/N共掺杂TiO2催化剂的禁带宽度分别为2.76、2.58、2.52 eV,证明Ce/N的协同作用使催化剂的红移更加明显。500℃煅烧后所得Ce(0.6%)-N-TiO2在500 W中压汞灯(波长范围260~570 nm)下,产氢速率达到120 μmol⋅h-1,是未掺杂TiO2的20倍。然而,某些掺杂离子常常成为光生电子-空穴对的复合中心,从而影响产氢效率。通过共掺杂可以有效抑制复合中心的形成。Niishiro等[52]研究发现紫外光下Ni-Ta-SrTiO3以及Ni-SrTiO3的产氢效率较单纯的SrTiO3低,主要是由于Ni3+成为电子空穴复合中心并捕获了光生电子造成的。可见光照下,SrTiO3并没有产氢能力,而Ni-Ta-SrTiO3及Ni-SrTiO3具有了产氢能力,且Ni-Ta-SrTiO3的产氢能力较Ni-SrTiO3的高。这主要是由于离子掺杂扩大了SrTiO3的可见光吸收能力,且Ni、Ta共掺杂后Ta5+起到了电荷补偿的作用,从而抑制了Ni3+电子空穴复合中心的形成。
|
图 4 金属(A、B)、非金属(C)离子掺杂改性改变催化剂能带结构示意图[19] Fig.4 Schematic diagram of band structure modification via metal (A,B) and (C) nonmetalion doping[19] |
助催化剂负载作为一种有效的催化剂表面修饰技术得到了广泛的研究。常见的光催化分解水产氢助催化剂主要有贵金属(Au、Pt、Rh、Ru、Pd等[53~56])、过渡金属氧化物(NiO、RuO2等[57, 58])、过渡金属硫化物(PdS、WS2等[59, 60])。对于光催化分解水产氢系统,助催化剂的作用包括以下几个方面[61, 62]:a. 促进基底催化剂内部光生电子和空穴分别向还原助催化剂和氧化助催化剂转移,从而加速催化剂内部电子空穴对的分离效率,提高光催化活性;b. 在基底催化剂表面为产氢和产氧反应提供活性位点;c. 增强催化剂的稳定性,降低催化反应活化能;d. 在助催化剂活性位点上产氢或产氧,可抑制逆反应(氢气和氧气结合重新形成水)的进行。Yan等[63]研究了不同助催化剂修饰CdS的光催化产氢性能。研究发现Pt/CdS的光催化产氢能力高于单纯的CdS催化剂,这是由于Pt的功函数大于CdS,Pt负载在CdS上后,在金属/催化剂界面形成肖特基势垒,促使CdS上光生电子向Pt转移,加速了电子空穴对的分离。PdS/CdS的光催化产氢性能较Pt/CdS更高,且其光催化稳定性比Pt/CdS更好。这主要是由于PdS作为一种氧化助催化剂,促进CdS内部光生空穴向PdS转移,从而加速了电子空穴对的分离。同时,PdS能有效抑制CdS催化剂的光腐蚀,增强基底催化剂的稳定性。而Pt-PdS/CdS的产氢效果较PdS/CdS进一步提高,且其同样具有较好的稳定性。这主要是由于Pt还原助催化剂和PdS氧化助催化剂的协同作用更有利于催化剂内部光生电子和空穴的分离,从而提高了光催化产氢效果。Wang等[64]制备了Ta3N5核壳结构催化剂,并在Ta3N5的内表面和外表面分别修饰了还原助催化剂Pt及氧化助催化剂IrO2。由于Ta3N5光生电子和光生空穴分别向Pt及IrO2转移,Ta3N5催化剂内部电子-空穴得到快速分离,该体系的可见光分解水性能得到显著提高。
3.2.2 表面等离子共振效应作为助催化剂的特例,某些碱性金属或贵金属(Au、Ag等)同时具有等离子体共振(Surface plasmon resonance,SPR)效应。当金属纳米粒子受到特定波长的光照射时,金属纳米颗粒内部电荷重新分配,同时金属表面的自由电子密度发生振荡,这种现象称为表面等离子体共振现象,也可称为局域表面等离子体共振(Localized surface plasmon resonance,LSPR)[65]。研究表明,等离子体贵金属与半导体光催化剂结合后能有效提升催化剂对可见光的吸收及光催化活性[66],且负载金属的形貌、大小均会影响光催化效率。目前,等离子体贵金属提升光催化产氢效率的机理主要有以下两个观点[67, 68]:a. 贵金属颗粒与染料敏化剂类似。光照时,离子体吸收光子产生的高能电子转移到催化剂的导带用于发生还原反应,从而使催化剂可见光化并提高参与光催化的光量子数,增强光催化效率(图 5 A所示)[69];b. SPR通过增强催化剂表面的电磁场强度,提高了基底催化剂内部电子-空穴对的生成率,同时光生电子通过肖特基势垒转移到负载金属上,从而提高了可见光下分解水产氢效率(图 5 B所示)[70]。Yuzawa等[71]制备了不同大小及形状的Au颗粒负载的TiO2催化剂,电镜表征说明Au以球状或杆状形式存在。由于电子传递速率更快,杆状Au纳米颗粒负载的TiO2具有更强的光催化产氢效率。论文中对于SPR的机理更倾向于上述第一个观点,即贵金属Au上的光生电子转移至TiO2的导带上并参与产氢反应。本课题组[72]研究了Au/N-TiO2的产氢效果,由于掺杂N和负载Au的协同作用,光照下(350~800 nm) Au/N-TiO2的产氢速率达到412.60 μmol⋅h-1,是N-TiO2的19.14倍,Au/TiO2的1.28倍。论文中对于SPR机理更倾向于第二种观点,即SPR效应增强了TiO2内部光生电荷生产率,从而增强了可见光产氢效率。
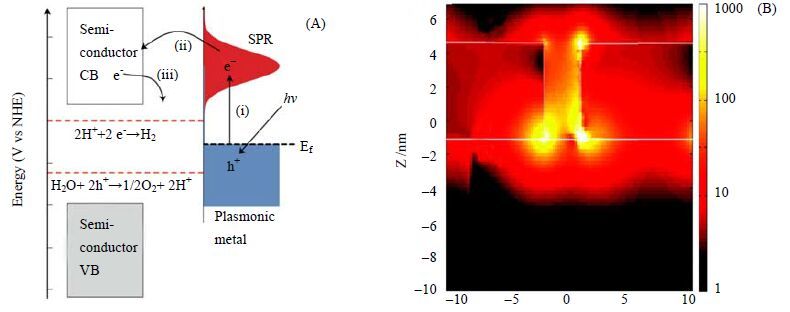
|
图 5 SPR 的电荷转移机理图(A)[67],有限差分时域法显示Au 的SPR 效应能增强TiO2 表面电磁场(B)[68] Fig.5 Mechanism of SPR-induced charge transfer with approximate energy levels(A)[67],Optical simulations showing enhanced electric field intensity at the interface of Au-TiO2(B)[68] |
通过制备一些具有特殊形貌的催化剂,从而获得一些新的性能,如:更大的比表面积、更多的活性位点、更稳定的催化性能等,同样是光催化领域的研究热点。常见的特殊形貌有纳米线[73]、纳米片[74]、纳米薄膜[75]、纳米管[76, 77]、纳米棒[78]、纳米纤维[79]、介孔结构[80, 81]、核壳结构[82, 83]、空心结构[84]等。Chaudhari等[85]制备了金盏花结构的N-TiO2,由于具有很大的比表面积,催化活性大大增强。Roy等[86]在无氟条件下利用二乙醇胺(DEA)作为封端剂及表面控制剂制备出了具有不同比例(101) 及(001) 晶面的锐钛矿相TiO2。由于(001) 、(101) 面分别被认为是氧化位点和还原位点,光生空穴和电子分别流向(001) 、(101) 晶面,从而加速了电子-空穴对的分离,提高了光催化活性(图 6)。
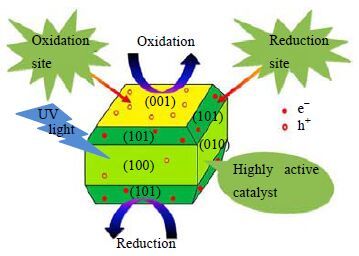
|
图 6 具有不同比例(001)和(101)晶面的长方体型 锐钛矿相TiO2[84] Fig.6 Scheme of TiO2 NCs with different exposed facets[84] |
某些硫族化合物具有较窄的禁带宽度,理论上其导带电子和价带空穴具有较强的氧化还原能力,然而由于其自身的光腐蚀作用,大大影响了可见光催化产氢效率。通过制备核壳结构催化剂,可以避免光腐蚀,提高光稳定性,使可见光得到有效利用。Xie等[87]在没有表面活性剂的情况下,通过一步水热法制备了CdS/ZnS核壳结构催化剂,ZnS壳具备孔状结构。由于CdS的光生空穴转移至ZnS导带中的Zn空位和间隙S上,而光生电子留在CdS上并参与产氢反应,形成了一种特殊的空间电荷分离体系。可见光下,CdS/ZnS核壳结构的产氢效率分别是ZnS和CdS的169和56倍,且即使在参与反应60 h之后,CdS/ZnS核壳结构的光催化产氢性能仍保持稳定(图 7)。
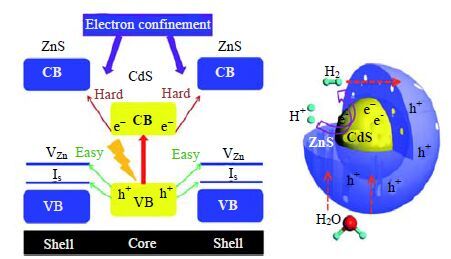
|
图 7 CdS/ZnS 核壳结构能带结构对比图和产氢机理图[85] Fig.7 Scheme of the band structure alignments and related photocatalytic reactions in the CdS/ZnS core/shell structure[85] |
两种具有不同能带结构的半导体复合后可能形成异质结构,由于不同半导体的导带和价带的差异,一方面使光生电子在一种半导体的导带上积累,另一方面使光生空穴在另一种半导体的价带上聚集,相应的提高了光生电子和空穴的分离率,扩展了光谱的吸收范围,从而表现出比单个半导体更好的稳定性及更高的光催化活性。Hou等[88]制备了具有α/β-Bi2O3异质结的Bi2O3纳米线。光照时,在内建电场力的作用下,α-Bi2O3的导带电子转移至β-Bi2O3的导带上,而β-Bi2O3的价带空穴转移至α-Bi2O3的价带上,从而阻止了光生电子-空穴对的复合,有效提高了Bi2O3的光催化性能(图 8)。Li等[89]用水热法制备了AgIn5S8/TiO2异质结纳米复合物,可见光照射下,AgIn5S8 (Eg≈1.76 eV)表面的光生电子迅速转移至TiO2表面,有效加速了催化剂表面电子空穴的分离,从而使可见光下产氢效率得到显著的提高。其中摩尔比为1:10的AgIn5S8/TiO2取得最佳产氢效果,是单纯AgIn5S8催化剂的7.7倍。
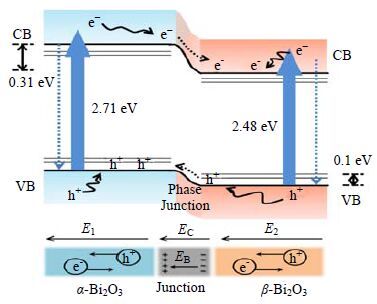
|
图 8 可见光下α/β-Bi2O3 异质结电子空穴分离示意图[86] Fig.8 In situ synthesis of α-β phase heterojunction on Bi2O3 nanowires with exceptional visible-light photocatalytic performance[86] |
由于能够调变催化剂的能带结构并获得更高的光催化效率,固溶体受到了广泛的关注[90, 91]。固溶体大多是由晶体结构相同、金属离子半径相近的宽禁带半导体和窄禁带半导体形成,通过调整宽禁带半导体和窄禁带半导体的配比,固溶体催化剂的禁带宽度及价带和导带位置可以在介于两种半导体之间的范围内进行调节。Kudo等[92]选用宽禁带的ZnS (3.5 eV)与窄禁带的AgInS2 (1.8 eV)制备出了不同能带结构的(AgIn)xZn2(1-x)S2固溶体。以Pt为助催化剂,SO32-与S2-作为空穴捕集剂,在可见光(λ > 420 nm)照射下,(AgIn)xZn2(1-x)S2固溶体的产氢速率远远大于ZnS及AgInS2。其中最优化的产氢催化剂为Pt(3%(wt)-(AgIn)0.22Zn1.56S2,在可见光下产氢速率为424 μmol⋅h-1(图 9)。本课题组[93]采用基本无产氢能力的窄禁带催化剂AgNbO3与只具有紫外光产氢能力的宽禁带催化剂SrTiO3制备了具有可见光产氢能力的(AgNbO3)1-x(SrTiO3)x (0 <x <1) 固溶体。随着x值的增大,固溶体的禁带宽度从2.65 eV持续增大至3.21 eV,CB位置不断变负,并在(AgNbO3)0.25(SrTiO3)0.75取得最佳可见光产氢速率。
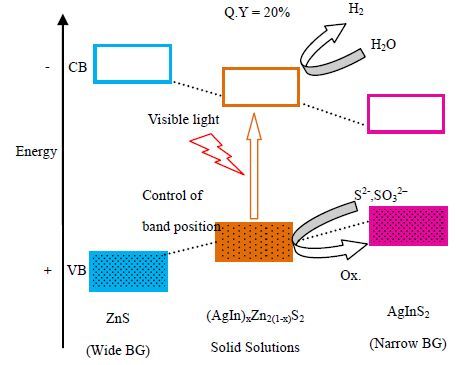
|
图 9 (AgIn)xZn2(1-x)S2 固溶体能带调节及光催化产氢图[90] Fig.9 Scheme of band structure modulation and photocatalytic hydrogen evolution of (AgIn)xZn2(1-x)S2 solid solutions[90] |
受自然界光合作用多电子转移机制的启发,Bard[94]于1979年提出了如图 10 B、C、D[95]所示的Z-scheme体系。该体系将窄禁带催化剂B和C通过电子传递物D(mediator)结合,可见光激发下,B的导带电子与C的价带空穴结合或者分别与加入的电子传递物(氧化还原剂)反应,而B的价带空穴氧化水产生O2,C的导带电子还原水产生H2,实现水的整体分解。Z-scheme体系与传统的光解水体系相比降低了对催化剂能带结构及激发催化剂所需光能的要求,能更有效地利用可见光;同时,Z-scheme结构抑制了电子-空穴对的复合,具有较高的光催化效率[96]。根据电子传递物的种类,Z-scheme体系可以分为离子对电子传递物Z-scheme体系、固态电子传递物Z-scheme体系以及不需电子传递物的Z-scheme体系。
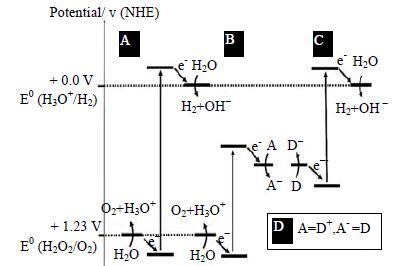
|
图 10 单一催化剂(A)及Z-scheme 体系(B、C、D)光解水示意图[93] Fig.10 Potential energy diagrams for photochemical water splitting at pH = 0: (A) single semiconductor system; (B) with an electron acceptor; (C) with an electron donor; (D) dual semiconductor system (z scheme) employing a redox shuttle[93] |
(a) 离子对电子传递物Z-scheme体系
传统的电子传递物是不同价态的离子对,如IO3-/I-和Fe3+/Fe2+。Domen等[95]以ZrO2/TaON作为产氢催化剂,Pt/WO3为产氧催化剂,IO3-/I-为电子传递物构建的Z-scheme体系在420 nm可见光下的表观量子产率达到6.3 %。光致发光光谱和电化学表征证明IO3-/I-与催化剂接触可高效传导电子、有效抑制电子-空穴对的复合,进而提高光催化效率。
(b) 固态电子传递物Z-scheme体系
相比于离子态电子传递物,固态电子传递物更有利于催化剂的回收且不会造成二次污染[98]。多种固态电子传递物被用于构建Z-scheme体系并取得了较高的电子传导效率。Yun等[99]建立了CdS-Au-TiO1.98C0.04全固态三组分Z-scheme体系,Au沉积于锐钛矿TiO1.98C0.04上,而Au和CdS形成半球状内核-外壳结构(图 11)。在大于420 nm的光照下,TiO1.98C0.04中的光生空穴参与氧化反应,光生电子通过Au传递至CdS的价带,与CdS的价带空穴结合,CdS的导带电子参与还原水反应产生氢气。电子沿着TiO2-Au-CdS的次序定向转移,从而提高了载流子的分离效率及光催化性能。
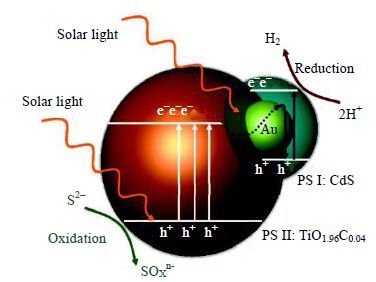
|
图 11 CdS-Au-TiO2 全固态三组分Z-Scheme 体系示意图[97] Fig.11 Electron-hole separation and transport at the visible-light-driven CdS-Au-TiO1.98C0.04 composite interface[97] |
(c) 不需电子传递物的Z-scheme体系
尽管电子传递物对电子在产氧催化剂和产氢催化剂之间的传递有重要作用,其存在也可能带来负面影响,如有色的电子传递物可干扰催化剂的光吸收[100]。因此,有研究者构建了无需外加电子传递物的Z-scheme体系。Jin等[101]采用高温煅烧联合水热法制备了大比表面积的g-C3N4。并将g-C3N4与WO3机械研磨混合,制备了g-C3N4/WO3 Z-scheme体系。光照下,WO3的光生电子与g-C3N4的光生空穴结合,从而促进了整个系统电子空穴的分离,增大了光催化效率。
3.7 修饰与改性组合技术光催化剂的修饰与改性研究过程中,为了进一步加速电子空穴分离效率,提高催化剂的光催化活性,常常采用催化剂修饰与改性组合技术,如:掺杂+助催化剂,掺杂+异质结,异质结+助催化剂等。Wang等[102]研究了P型半导体/金属混合催化剂Pd/Cu2O的光催化活性。Cu2O是一种P型半导体,其具有(100) 和(111) 晶面,光生电子和空穴分别向(111) 和(100) 晶面积累,从而实现了电荷的空间分离。Cu2O的(100) 晶面的功函数较小,当在其表面负载Pd后,光生空穴并不能通过肖特基势垒转移至Cu2O的(100) 晶面上。而由于Cu2O的(111) 晶面具有较大的功函数,其与Pd结合后,可形成肖特基势垒,使得光生空穴向(111) 晶面转移。通过调节Pd的比例,可以使Cu2O上的电子和Pd上的空穴达到动态平衡。在肖特基势垒和空间电荷分离协同作用下,光生电子空穴分离效率大大提高。Li等[103]研究了双助催化剂负载的CdS/ZnS核/壳结构催化剂。通过制备ZnS壳,抑制了CdS的光腐蚀,并钝化了CdS表面深陷阱,加速了光还原产氢效率。同时他们还发现,Pt、Ni是有效的还原助催化剂(RC),而PdS和PbS是有效的氧化助催化剂(OC)。在核壳结构外负载双助催化剂体系,不仅有效促进了电子-空穴对分离,并为氧化和还原反应提供了活性位点(图 12)。
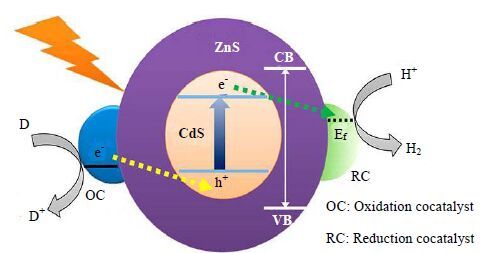
|
图 12 双助催化剂负载的CdS/ZnS 核/壳结构纳米晶体上电子-空穴转 移和分离示意图[101] Fig.12 Schematic description of the transfer and separation of the photogenerated electrons and holes from dual cocatalysts loaded CdS/ZnS core/shell nanocrystals[101] |
针对传统光催化产氢催化剂稳定性差、可见光利用率低、产氢速率低等问题,科学家们在催化剂的修饰与改性方面进行了大量的研究,如:掺杂改性、贵金属负载、制备特殊形貌催化剂等,并取得了许多重要的研究成果。但是,光催化分解水产氢效率,特别是可见光下分解水产氢效率仍远未达到工业应用的要求。为了进一步提高光催化产氢效率、克服光催化分解水领域的诸多问题,在催化剂制备方面,可以从以下几个方面入手:(1) 不断探索创新,研究改善催化剂晶型、结晶度、表面活性、形貌等的催化剂制备方法,开发新型可见光响应催化剂,提高光催化产氢效率。(2) 目前,催化剂的修饰与改性技术在提高催化剂光催化性能方面成效显著,但是部分技术仍存在一些缺陷,如:催化剂掺杂常常会引进电子空穴复合中心。因而,需要进一步研究新型的催化剂修饰与改性技术。(3) 催化剂修饰与改性组合技术能够有效提高催化剂内部电荷分离效率、改善光催化性能,如异质结+助催化剂能够进一步提高电子空穴对的分离效率。因而,可以进一步研究催化剂修饰与改性组合技术,以提高光催化剂可见光产氢性能。(4) 结合理论计算等辅助手段研究催化剂的能带结构及组成,用于预测潜在的高效光催化剂,同时用于光催化反应机理探索。随着研究的进一步深入,光催化剂存在的缺点将逐渐被克服,光催化产氢效率将不断提高,相信在未来的能源市场上光催化产氢将占有一席之地。
| [1] | Kado Y, Lee C Y, Lee K . Enhanced water splitting activity of M-doped Ta3N5[J]. Chemical Communications , 2012, 48 (69) : 8685-8687 DOI:10.1039/c2cc33822j |
| [2] | Wang D, Pierre A, Kibria M G . Wafer-level photocatalytic water splitting on GaN nanowire arrays grown by molecular beam epitaxy[J]. Nano Letters , 2011, 11 (6) : 2353-2357 DOI:10.1021/nl2006802 |
| [3] | Xiang Q, Yu J . Graphene-based photocatalysts for hydrogen generation[J]. Journal of Physical Chemistry Letters , 2013, 4 (5) : 753-759 DOI:10.1021/jz302048d |
| [4] | Maeda K, Domen K . Photocatalytic water splitting:recent progress and future challenges[J]. Journal of Physical Chemistry Letters , 2010, 1 (18) : 2655-2661 DOI:10.1021/jz1007966 |
| [5] | Ma Y, Wang X, Jia Y . Titanium dioxide-based nanomaterials for photocatalytic fuel generations[J]. Chemical Reviews , 2014, 114 (19) : 9987-10043 DOI:10.1021/cr500008u |
| [6] | Huang S, Lin Y, Yang J H, et al. CdS-based semiconductor photocatalysts for hydrogen production from water splitting under solar light[C]. In Nanotechnology for Sustainable Energy. Acs Symposium Series, American Chemical Society:Washington, D C, 2013, 1140:219-241. |
| [7] | Adeli B, Taghipour F . A review of synthesis techniques for gallium-zinc oxynitride solar-activated photocatalyst for water splitting[J]. Ecs Journal of Solid State Science and Technology , 2013, 2 (7) : Q118-Q126 DOI:10.1149/2.022307jss |
| [8] | Zhang N, Zhang Y, Xu Y J . Recent progress on graphene-based photocatalysts:current status and future perspectives[J]. Nanoscale , 2012, 4 (19) : 5792-5813 DOI:10.1039/c2nr31480k |
| [9] | CHU Zeng-yong(楚增勇), YUAN Bo(原博), YAN Ting-nan(颜廷楠) . Recent progress in photocatalysis of g-C3N4(g-C3N4光催化性能的研究进展)[J]. Journal of Inorganic Materials(无机材料学报) , 2014, 29 (8) : 785-794 DOI:10.15541/jim20130633 |
| [10] | Hisatomi T, Kubota J, Domen K . Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting[J]. Chemical Society Reviews , 2014, 43 (22) : 7520-7535 DOI:10.1039/C3CS60378D |
| [11] | Shen S, Mao S S . Nanostructure designs for effective solar-to-hydrogen conversion[J]. Nanophotonics , 2012, 1 (1) : 31-50 |
| [12] | Kudo A, Miseki Y . Heterogeneous photocatalyst materials for water splitting[J]. Chemical Society Reviews , 2009, 38 (1) : 253-278 DOI:10.1039/B800489G |
| [13] | WEN Fu-yu(温福宇), YANG Jin-hui(杨金辉), SONG Xu(宋旭) . Photocatalytic hydrogen production utilizing solar energy(太阳能光催化制氢研究进展)[J]. Progress in Chemistry(化学进展) , 2009, 21 (11) : 2286-2302 |
| [14] | YAN Shi-cheng(闫世成), LUO Wen-jun(罗文俊), LI Zhao-sheng(李朝升) . Progress in research of novel photocatalytic materials(新型光催化材料探索和研究进展)[J]. Materials China(中国材料进展) , 2010, 29 (1) : 1-9 |
| [15] | CHEN Wei(陈威), WANG Hui(王慧), YANG Yu(杨俞) . Progress in research of photocatalytic hydrogen production from overall water splitting(光催化完全分解水制氢研究进展)[J]. Chemical Research(化学研究) , 2014, 25 (2) : 201-208 |
| [16] | Xing Z, Zong X, Pan J . On the engineering part of solar hydrogen production from water splitting:photoreactor design[J]. Chemical Engineering Science , 2013, 104 : 125-146 DOI:10.1016/j.ces.2013.08.039 |
| [17] | Preethi V, Kanmani S . Photocatalytic hydrogen production[J]. Materials Science In Semiconductor Processing , 2013, 16 (3) : 561-575 DOI:10.1016/j.mssp.2013.02.001 |
| [18] | Linsebigler A L, Lu G, Yates Jr J T . Photocatalysis on TiO2 surfaces:principles, mechanisms, and selected results[J]. Chemical Reviews , 1995, 95 (3) : 735-758 DOI:10.1021/cr00035a013 |
| [19] | Chen X, Shen S, Guo L . Semiconductor-based photocatalytic hydrogen generation[J]. Chemical Reviews , 2010, 110 (11) : 6503-6570 DOI:10.1021/cr1001645 |
| [20] | Yu J, Qi L, Jaroniec M . Hydrogen production by photocatalytic water splitting over Pt/TiO2 nanosheets with exposed (001) facets[J]. Journal of Physical Chemistry C , 2010, 114 (30) : 13118-13125 DOI:10.1021/jp104488b |
| [21] | Liu S-H, Wang H P . Photocatalytic generation of hydrogen on Zr-MCM-41[J]. International Journal of Hydrogen Energy , 2002, 27 (9) : 859-862 DOI:10.1016/S0360-3199(01)00190-2 |
| [22] | Ouyang S, Tong H, Umezawa N . Surface-alkalinization-induced enhancement of photocatalytic H2 evolution over SrTiO3-based photocatalysts[J]. Journal of The American Chemical Society , 2012, 134 (4) : 1974-1977 DOI:10.1021/ja210610h |
| [23] | Wang X, Xu Q, Li M, et al Shen S . Photocatalytic overall water splitting promoted by an α-β phase junction on Ga2O3[J]. Angewandte Chemie-International Edition , 2012, 51 (52) : 13089-13092 DOI:10.1002/anie.201207554 |
| [24] | Primo A, Marino T, Corma A . Efficient visible-light photocatalytic water splitting by minute amounts of gold supported on nanoparticulate CeO2 obtained by a biopolymer templating method[J]. Journal of The American Chemical Society , 2011, 133 (18) : 6930-6933 DOI:10.1021/ja2011498 |
| [25] | Kudo A, Sekizawa M . Photocatalytic H2 evolution under visible light irradiation on Ni-doped ZnS photocatalyst[J]. Chemical Communications , 2000, 15 : 1371-1372 |
| [26] | Maeda K, Takata T, Hara M . GaN:ZnO solid solution as a photocatalyst for visible-light-driven overall water splitting[J]. Journal of The American Chemical Society , 2005, 127 (23) : 8286-8287 DOI:10.1021/ja0518777 |
| [27] | Serpone N, Pelizzetti E . Photocatalysis:fundamentals and applications[M]. New York: John Wiley & Sons, 1989 . |
| [28] | CHENG Zhuo-wei(成卓韦), ZHOU Ling-jun(周灵俊), YU Jian-ming(於建明) . Mechanism study on gaseous α-pinene photocatalyzed by lanthanum-doped titanium dioxide nanotubes(镧掺杂TiO2纳米管对α-蒎烯光催化性能及催化机理研究)[J]. Journal of Chemical Engineering of Chinese Universities(高校化学工程学报) , 2015, 29 (2) : 320-327 |
| [29] | XUE Shou-qing(薛守庆) . Microwave-assisted synthesis of Cu(OH)2/ZnO photocatalyst and its photocatalysis(微波辅助纳米Cu(OH)2/ZnO复合材料的合成与光催化研究)[J]. Journal of Chemical Engineering of Chinese Universities(高校化学工程学报) , 2014, 28 (1) : 150-155 |
| [30] | O ng, J W, Tan LL . Highly reactive {001} facets of TiO2 based composites:synthesis, formation mechanism and characterization[J]. Nanoscale , 2014, 6 (4) : 1946-2008 DOI:10.1039/c3nr04655a |
| [31] | Yuan YP, Xu WT, Yin LS . Large impact of heating time on physical properties and photocatalytic H2 production of g-C3N4 nanosheets synthesized through urea polymerization in Ar atmosphere[J]. International Journal of Hydrogen Energy , 2013, 38 (30) : 13159-13163 DOI:10.1016/j.ijhydene.2013.07.104 |
| [32] | Zhang Y, Liu J, Wu G . Porous graphitic carbon nitride synthesized via direct polymerization of urea for efficient sunlight-driven photocatalytic hydrogen production[J]. Nanoscale , 2012, 4 (17) : 5300-5303 DOI:10.1039/c2nr30948c |
| [33] | Anderson , J A . Simultaneous photocatalytic degradation of nitrate and oxalic acid over gold promoted titania[J]. Catalysis Today , 2012, 181 : 171-176 DOI:10.1016/j.cattod.2011.05.027 |
| [34] | Berr M J, Wagner P, Fischbach S . Hole scavenger redox potentials determine quantum efficiency and stability of Pt-decorated CdS nanorods for photocatalytic hydrogen generation[J]. Applied Physics Letters , 2012 |
| [35] | Jang J S, Joshi U A, Lee J S . Solvothermal synthesis of CdS nanowires for photocatalytic hydrogen and electricity production[J]. Journal of Physical Chemistry C , 2007, 111 (35) : 13280-13287 DOI:10.1021/jp072683b |
| [36] | Tsuji I, Kato H, Kudo A . Visible-light-induced H2 evolution from an aqueous solution containing sulfide and sulfite over a ZnS-CuInS2-AgInS2 solid-solution photocatalyst[J]. Angewandte Chemie-International Edition , 2005, 117 (23) : 3631-3634 |
| [37] | Khan M A, Woo S I, Yang O . Hydrothermally stabilized Fe(III) doped titania active under visible light for water splitting reaction[J]. International Journal of Hydrogen Energy , 2008, 33 (20) : 5345-5351 DOI:10.1016/j.ijhydene.2008.07.119 |
| [38] | Wang D, Ye J, Kako T . Photophysical and photocatalytic properties of SrTiO3 doped with Cr cations on different sites[J]. Journal of Physical Chemistry B , 2006, 110 (32) : 15824-15830 DOI:10.1021/jp062487p |
| [39] | Arai T, Senda S, Sato Y . Cu-doped ZnS hollow particle with high activity for hydrogen generation from alkaline sulfide solution under visible light[J]. Chemistry of Materials , 2008, 20 (5) : 1997-2000 DOI:10.1021/cm071803p |
| [40] | Dholam R, Patel N, Adami M . Hydrogen production by photocatalytic water-splitting using Cr-or Fe-doped TiO2 composite thin films photocatalyst[J]. International Journal of Hydrogen Energy , 2009, 34 (13) : 5337-5346 DOI:10.1016/j.ijhydene.2009.05.011 |
| [41] | Kim D H, Choi D K, Kim S J . The effect of phase type on photocatalytic activity in transition metal doped TiO2 nanoparticles[J]. Catalysis Communications , 2008, 9 (5) : 654-657 DOI:10.1016/j.catcom.2007.07.017 |
| [42] | Devi L G, Kumar S G, Murthy B N . Influence of Mn2+ and Mo6+ dopants on the phase transformations of TiO2 lattice and its photocatalytic activity under solar illumination[J]. Catalysis Communications , 2009, 10 (6) : 794-798 DOI:10.1016/j.catcom.2008.11.041 |
| [43] | Tian B, Li C, Gu F . Flame sprayed V-doped TiO2 nanoparticles with enhanced photocatalytic activity under visible light irradiation[J]. Chemcical Engineering Journal , 2009, 151 (1) : 220-227 |
| [44] | Yin C, Zhu S, Chen Z . One step fabrication of C-doped BiVO4 with hierarchical structures for a high-performance photocatalyst under visible light irradiation[J]. Journalof Materials Chemistry A , 2013, 1 (29) : 8367-8378 DOI:10.1039/c3ta11833a |
| [45] | Yang G, Jiang Z, Shi H . Preparation of highly visible-light active N-doped TiO2 photocatalyst[J]. Journalof Materials Chemistry , 2010, 20 (25) : 5301-5309 DOI:10.1039/c0jm00376j |
| [46] | Ma D, Xin Y, Gao M . Fabrication and photocatalytic properties of cationic and anionic S-doped TiO2 nanofibers by electrospinning[J]. Applied Catalysis B:Environmental , 2014, 147 : 49-57 DOI:10.1016/j.apcatb.2013.08.004 |
| [47] | Wang J, Tafen D N, Lewis J P . Origin of photocatalytic activity of nitrogen-doped TiO2 nanobelts[J]. Journal of The American Chemical Society , 2009, 131 (34) : 12290-12297 DOI:10.1021/ja903781h |
| [48] | Di Valentin C, Finazzi E, Pacchioni G . N-doped TiO2:theory and experiment[J]. Chemical Physics , 2007, 339 (1) : 44-56 |
| [49] | Livraghi S, Paganini M C, Giamello E . Origin of photoactivity of nitrogen-doped titanium dioxide under visible light[J]. Journal of The American Chemical Society , 2006, 128 (49) : 15666-15671 DOI:10.1021/ja064164c |
| [50] | Wang F, Di Valentin C, Pacchioni G . Doping of WO3 for photocatalytic water splitting:hints from density functional theory[J]. Journal of Physical Chemistry C , 2012, 116 (16) : 8901-8909 DOI:10.1021/jp300867j |
| [51] | Sun X, Liu H, Dong J . Preparation and characterization of Ce/N-codoped TiO2 particles for production of H2 by photocatalytic splitting water under visible light[J]. Catalysis Letters , 2010, 135 (3-4) : 219-225 DOI:10.1007/s10562-010-0302-7 |
| [52] | Niishiro R, Kato H, Kudo A . Nickel and either tantalum or niobium-codoped TiO2 and SrTiO3 photocatalysts with visible-light response for H2 or O2 evolution from aqueous solutions[J]. Physical Chemistry Chemical Physics , 2005, 7 (10) : 2241-2245 DOI:10.1039/b502147b |
| [53] | Zhang J, Wang Y, Zhang J . Enhanced photocatalytic hydrogen production activities of Au-loaded ZnS flowers[J]. Acs Applied Materials & Interfaces , 2013, 5 (3) : 1031-1037 |
| [54] | Ma B J, Kim J S, Choi C H . Enhanced hydrogen generation from methanol aqueous solutions over Pt/MoO3/TiO2 under ultraviolet light[J]. International Journal of Hydrogen Energy , 2013, 38 (9) : 3582-3587 DOI:10.1016/j.ijhydene.2012.12.142 |
| [55] | Okamoto Y, Ida S, Hyodo J . Synthesis and photocatalytic activity of rhodium-doped calcium niobate nanosheets for hydrogen production from a water/methanol system without cocatalyst loading[J]. Journal of The American Chemical Society , 2011, 133 (45) : 18034-18037 DOI:10.1021/ja207103j |
| [56] | Tanabe I, Ozaki Y . Consistent changes in electronic states and photocatalytic activities of metal (Au, Pd, Pt)-modified TiO2 studied by far-ultraviolet spectroscopy[J]. Chemical Communications , 2014, 50 (17) : 2117-2119 DOI:10.1039/c3cc48446g |
| [57] | Jeong H, Kim T, Kim D . Hydrogen production by the photocatalytic overall water splitting on NiO/Sr3Ti2O7:effect of preparation method[J]. International Journal of Hydrogen Energy , 2006, 31 (9) : 1142-1146 DOI:10.1016/j.ijhydene.2005.10.005 |
| [58] | Kadowaki H, Saito N, Nishiyama H . Overall splitting of water by RuO2-loaded PbWO4 photocatalyst with d10s2-d0 configuration[J]. Journal of Physical Chemistry C , 2007, 111 (1) : 439-444 DOI:10.1021/jp065655m |
| [59] | Yang J, Yan H, Wang X . Roles of cocatalysts in Pt-PdS/CdS with exceptionally high quantum efficiency for photocatalytic hydrogen production[J]. Journal of Catalysis , 2012, 290 : 151-157 DOI:10.1016/j.jcat.2012.03.008 |
| [60] | Zong X, Han J, Ma G . Photocatalytic H2 evolution on CdS loaded with WS2 as cocatalyst under visible light irradiation[J]. Journal of Physical Chemistry C , 2011, 115 (24) : 12202-12208 DOI:10.1021/jp2006777 |
| [61] | Ni M, Leung M K, Leung D Y . A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production[J]. Renewable & Sustainable Energy Reviews , 2007, 11 (3) : 401-425 |
| [62] | Yang J, Wang D, Han H . Roles of cocatalysts in photocatalysis and photoelectrocatalysis[J]. Accounts of Chemical Research , 2013, 46 (8) : 1900-1909 DOI:10.1021/ar300227e |
| [63] | Yan H, Yang J, Ma G . Visible-light-driven hydrogen production with extremely high quantum efficiency on Pt-PdS/CdS photocatalyst[J]. Journal of Catalysis , 2009, 266 (2) : 165-168 DOI:10.1016/j.jcat.2009.06.024 |
| [64] | Wang D, Hisatomi T, Takata T . Core/shell photocatalyst with spatially separated Co-catalysts for efficient reduction and oxidation of water[J]. Angewandte Chemie-International Edition , 2013, 52 (43) : 11252-11256 DOI:10.1002/anie.v52.43 |
| [65] | Zhou X, Liu G, Yu J . Surface plasmon resonance-mediated photocatalysis by noble metal-based composites under visible light[J]. Journalof Materials Chemistry , 2012, 22 : 21337-21354 DOI:10.1039/c2jm31902k |
| [66] | Tanaka A, Ogino A, Iwaki M . Gold-titanium(IV) oxide plasmonic photocatalysts prepared by a colloid-photodeposition method:correlation between physical properties and photocatalytic activities[J]. Langmuir , 2012, 28 (36) : 13105-13111 DOI:10.1021/la301944b |
| [67] | Wang P, Huang B, Dai Y . Plasmonic photocatalysts:harvesting visible light with noble metal nanoparticles[J]. Physical Chemistry Chemical Physics , 2012, 14 (28) : 9813-9825 DOI:10.1039/c2cp40823f |
| [68] | Chen J-J, Wu J C S, Wu P C . Plasmonic photocatalyst for H2 evolution in photocatalytic water splitting[J]. Journal of The American Chemical Society , 2011, 115 (1) : 210-216 |
| [69] | Linic S, Christopher P, Ingram D B . Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy[J]. Nature Materials , 2011, 10 (12) : 911-921 DOI:10.1038/nmat3151 |
| [70] | Liu Z, Hou W, Pavaskar P . Plasmon resonant enhancement of photocatalytic water splitting under visible illumination[J]. Nano Letters , 2011, 11 : 1111-1116 DOI:10.1021/nl104005n |
| [71] | Yuzawa H, Yoshida T, Yoshida H . Gold nanoparticles on titanium oxide effective for photocatalytic hydrogen formation under visible light[J]. Applied Catalysis B:Environmental , 2012, 115 : 294-302 |
| [72] | Zhao W, Ai Z, Dai J . Enhanced photocatalytic activity for H2 evolution under irradiation of UV-Vis light by Au-modified nitrogen-doped TiO2[J]. Plos ONE , 2014, 9 (8) : e103671 DOI:10.1371/journal.pone.0103671 |
| [73] | Yu J, Yu Y, Zhou P, et al. Morphology-dependent photocatalytic H2 production activity of CdS[J]. Applied Catalysis B:Environmental, 2014, 156-157:184-191. |
| [74] | Yang S, Gong Y, Zhang J . Exfoliated graphitic carbon nitride nanosheets as efficient catalysts for hydrogen evolution under visible light[J]. Advanced Materials , 2013, 25 (17) : 2452-2456 DOI:10.1002/adma.v25.17 |
| [75] | WU Ya-hui(吴亚惠), LONG Ming-ce(龙明策), CAI Wei-ming(蔡伟民) . Novel synthesis and property of TiO2 nano film photocatalyst with mixed phases(混晶纳米TiO2薄膜光催化剂的新型制备方法及性能研究)[J]. Journal of Chemical Engineering of Chinese Universities(高校化学工程学报) , 2010, 24 (6) : 1005-1010 |
| [76] | Wang J, Cao F, Bian Z . Ultrafine single-crystal TiOF2 nanocubes with mesoporous structure, high activity and durability in visible light driven photocatalysis[J]. Nanoscale , 2014, 6 (2) : 897-902 DOI:10.1039/C3NR04489K |
| [77] | LAN Yu-wei(兰宇卫), ZHOU Li-ya(周立亚), TONG-Zhang-fa (童张法) . Fabrication and property investigation of the highly ordered TiO2 nanotube arrays(高度有序TiO2纳米管阵列的制备及其光催化性能研究)[J]. Journal of Chemical Engineering of Chinese Universities(高校化学工程学报) , 2011, 25 (3) : 507-512 |
| [78] | Zhang L J, Zheng R, Li S . Enhanced photocatalytic H2 generation on cadmium sulfide nanorods with cobalt hydroxide as cocatalyst and insights into their photogenerated charge transfer properties[J]. Acs Applied Materials & Interfaces , 2014, 6 (16) : 13406-13412 |
| [79] | Suresh Kumar P, Jayaraman S, Subramanian S . Hierarchical electrospun nanofibers for energy harvesting, production and environmental remediation[J]. Energy &Environmental Science , 2014, 7 : 3192-3222 |
| [80] | Zhou W, Li W, Wang JQ . Ordered mesoporous black TiO2 as highly efficient hydrogen evolution photocatalyst[J]. Journal of The American Chemical Society , 2014, 136 (26) : 9280-9283 DOI:10.1021/ja504802q |
| [81] | Dai F, Zai J, Yi R . Bottom-up synthesis of high surface area mesoporous crystalline silicon and evaluation of its hydrogen evolution performance[J]. Nature Communications , 2014, 5 : 4605/1-4605/11 |
| [82] | Dong R, Tian B, Zhang J . AgBr@Ag/TiO2 core-shell composite with excellent visible light photocatalytic activity and hydrothermal stability[J]. Catalysis Communications , 2013, 38 : 16-20 DOI:10.1016/j.catcom.2013.04.006 |
| [83] | Zhang X, Zhu Y, Yang X . Enhanced visible light photocatalytic activity of interlayer-isolated triplex Ag@SiO2@TiO2 core-shell nanoparticles[J]. Nanoscale , 2013, 5 (8) : 3359-3366 DOI:10.1039/c3nr00044c |
| [84] | Dinh C-T, Yen H, Kleitz F . Three-dimensional ordered assembly of thin-shell Au/TiO2 hollow nanospheres for enhanced visible-light-driven photocatalysis[J]. Angewandte Chemie-International Edition , 2014, 53 (26) : 6618-6623 DOI:10.1002/anie.201400966 |
| [85] | Chaudhari N S, Warule S S, Dhanmane S A . Nanostructured N-doped TiO2 marigold flowers for an efficient solar hydrogen production from H2S[J]. Nanoscale , 2013, 5 (19) : 9383-9390 DOI:10.1039/c3nr02975a |
| [86] | Roy N, Sohn Y, Pradhan D . Synergy of low-energy {101} and high-energy {001} TiO2 crystal facets for enhanced photocatalysis[J]. ACS Nano , 2013, 7 (3) : 2532-2540 DOI:10.1021/nn305877v |
| [87] | Xie Y P, Yu Z B, Liu G . CdS-mesoporous ZnS core-shell particles for efficient and stable photocatalytic hydrogen evolution under visible light[J]. Energy &Environmental Science , 2014, 7 (6) : 1895-1901 |
| [88] | Hou J, Yang C, Wang Z, et al. In situ synthesis of α-β phase heterojunction on Bi2O3 nanowires with exceptional visible-light photocatalytic performance[J]. Applied Catalysis B:Environmental, 2013, 142-143:504-511. |
| [89] | Li K, Chai B, Peng T . Preparation of AgIn5S8/TiO2 heterojunction nanocomposite and its enhanced photocatalytic H2production property under visible light[J]. ACS Catalysis , 2013, 3 (2) : 170-177 DOI:10.1021/cs300724r |
| [90] | Liu H, Yuan J, Jiang Z . Novel photocatalyst of V-based solid solutions for overall water splitting[J]. Journal of Materials Chemistry , 2011, 21 (41) : 16535-16543 DOI:10.1039/c1jm11809a |
| [91] | Wang Q, An N, Chen W . Photocatalytic water splitting into hydrogen and research on synergistic of Bi/Sm with solid solution of Bi-Sm-V photocatalyst[J]. International Journal of Hydrogen Energy , 2012, 37 (17) : 12886-12892 DOI:10.1016/j.ijhydene.2012.05.080 |
| [92] | Tsuji I, Kato H, Kobayashi H . Photocatalytic H2 evolution reaction from aqueous solutions over band structure-controlled (AgIn)xZn2(1-x)S2 solid solution photocatalysts with visible-light response and their surface nanostructures[J]. Journal of The American Chemical Society , 2004, 126 (41) : 13406-13413 DOI:10.1021/ja048296m |
| [93] | Zhao W, Ai Z, Zhu X . Visible-light-driven photocatalytic H2 evolution from water splitting with band structure tunable solid solution (AgNbO3)1-x(SrTiO3)x[J]. International Journal of Hydrogen Energy , 2014, 39 (15) : 7705-7712 DOI:10.1016/j.ijhydene.2014.03.102 |
| [94] | Bard A J . Photoelectrochemistry and heterogeneous photocatalysis at semiconductors[J]. Journal of Photochemistry , 1979, 10 (1) : 59-75 DOI:10.1016/0047-2670(79)80037-4 |
| [95] | Osterloh F E . Inorganic materials as catalysts for photochemical splitting of water[J]. Chemistry of Materials , 2008, 20 (1) : 35-54 DOI:10.1021/cm7024203 |
| [96] | Ma S S K, Hisatomi T, Domen K . Hydrogen production by photocatalytic water splitting[J]. Journal of the Japan Petroleum Institute , 2013, 56 (5) : 280-287 DOI:10.1627/jpi.56.280 |
| [97] | Maeda K, Higashi M, Lu D . Efficient nonsacrificial water splitting through two-step photoexcitation by visible light using a modified oxynitride as a hydrogen evolution photocatalyst[J]. Journal of The American Chemical Society , 2010, 132 (16) : 5858-5868 DOI:10.1021/ja1009025 |
| [98] | Iwase A, Ng Y H, Ishiguro Y . Reduced graphene oxide as a solid-state electron mediator in Z-scheme photocatalytic water splitting under visible light[J]. Journal of The American Chemical Society , 2011, 133 (29) : 11054-11057 DOI:10.1021/ja203296z |
| [99] | Yun H J, Lee H, Kim N D . A combination of two visible-light responsive photocatalysts for achieving the Z-scheme in the solid state[J]. ACS Nano , 2011, 5 (5) : 4084-4090 DOI:10.1021/nn2006738 |
| [100] | Kudo A . Z-scheme photocatalyst systems for water splitting under visible light irradiation[J]. MRS Bulletin , 2011, 36 (1) : 32-38 DOI:10.1557/mrs.2010.3 |
| [101] | Jin Z, Murakami N, Tsubota T, et al. Complete oxidation of acetaldehyde over a composite photocatalyst of graphitic carbon nitride and tungsten(VI) oxide under visible-light irradiation[J]. Applied Catalysis B:Environmental, 2014, 150-151:479-485. |
| [102] | Wang L, Ge J, Wang A . Designing p-type semiconductor-metal hybrid structures for improved photocatalysis[J]. Angewandte Chemie-International Edition , 2014, 53 (20) : 5107-5111 |
| [103] | Huang L, Wang X, Yang J . Dual cocatalysts loaded type I CdS/ZnS core/shell nanocrystals as effective and stable photocatalysts for H2 evolution[J]. Journal of Physical Chemistry C , 2013, 117 (22) : 11584-11591 DOI:10.1021/jp400010z |




