水污染是严重的环境问题,其治理极具挑战性,也是目前研究的热点和重点之一[1-4]。大多数有机染料都属于有毒污染物,对水体环境造成严重危害,其中罗丹明B(RhB)是一种广泛应用于纺织、印刷、皮革、医药、食品等行业的三苯甲烷类染料,具有强致癌性和致突变性,会引起诸如恶心、出血、癌症等水传播疾病,是水污染的主要来源之一,彻底去除和降解水体中此类有机污染物至关重要[5-7]。
催化降解是一类非常高效的去除水中有机污染物的方法[7]。金属和半导体材料在催化方面有着良好的应用前景[8-13],常见的贵金属Pd、Pt和Au等具有优异的催化性能,但其资源较稀缺,价格昂贵,且纳米粒子比表面积较大,作为催化剂使用时容易发生团聚。过渡金属Cu价格远低于Pd、Pt、Au等贵金属,是替代贵金属催化剂的理想材料之一,但是Cu颗粒也存在空气中易氧化、颗粒易团聚和催化活性较低等缺点[14-17]。将Cu颗粒负载在具有大比表面积的载体上来提高Cu的分散性、稳定性和催化活性是非常重要的研究方向。石墨烯是由sp2杂化的碳原子紧密排列成的单原子层二维蜂窝状结构,具有极大的比表面积(约2 630 m2·g-1),极高的电子迁移率(200 000 cm-2·v-1·s-1)以及高导热系数(约5 000 W·m-1·K-1)[18-23]。由于其优异的性能,石墨烯受到广泛关注[24-25]。已有研究将金属、金属氧化物、半导体及磁性纳米材料诸如Pd、Pt、Au、TiO2及Fe3O4等负载在石墨烯片层,以提高金属催化剂的活性,并减少贵金属的消耗[26-29]。文献报道的石墨烯负载金属纳米材料粒径一般在十几到几百个纳米[6],考虑到纳米材料的量子尺寸效应,合成超小尺寸的金属纳米材料正日益受到人们的关注[30],如LI等[31]选择性地将粒径3~5 nm的纳米贵金属(Pt、Pd、Pt@Pd)负载在Fe3O4@GO载体上,获得多功能纳米催化材料。但关于超小尺寸Cu纳米复合材料的制备及还原有机染料的催化性能研究较少。
本文对氧化石墨烯(GO)进行改性,以功能化的石墨烯为模板负载纳米铜,采用一步还原法,将Cu颗粒负载在GO片层上的同时GO被还原为rGO,制备超小粒径的Cu@rGO纳米复合材料,以RhB为模拟污染物,考察不同原料配比的Cu@rGO纳米复合材料在NaBH4存在下的催化降解性能。
2 实验 2.1 实验材料与仪器五水硫酸铜、醋酸、氢氧化钠、次磷酸钠、乙醇、NaBH4及RhB购于国药集团化学试剂有限公司,上述试剂均为分析纯;聚丙烯酰胺(PAM)购于烟台双双化工有限公司;氧化石墨烯(GO)采用改进Hummers法自制[32-33]。
样品物相分析用德国Bruker公司D8 X射线衍射仪(扫描范围2θ=10°~80°,扫描速度0.2 °·min-1);表面分析用美国ThermoFisher公司ESCALAB 250Xi光电子能谱仪。形貌分析用日本JEOL公司JEM-2100透射电子显微镜;光学测试通过Thermo Fisher科技公司Evolution 201紫外-可见分光光度计进行。
2.2 材料制备称取计量的GO和0.240 0 g PAM,超声分散于16 mL蒸馏水中,滴加6.2 mL醋酸,60 ℃加热搅拌1 h并冷却至室温;按照GO:Cu(mol:mol)=1:1、1:3、1:5的比例分别称量一定量的五水硫酸铜,溶于80 mL蒸馏水中,调节溶液pH=9,再加入0.73 g次磷酸钠,80 ℃水浴搅拌4 h,冷却后将溶液在40 ℃下旋蒸除去溶剂,所得样品分别用蒸馏水和乙醇洗涤数次后,于40 ℃真空干燥箱中干燥12 h,得到不同铜含量的Cu@rGO纳米复合材料和纯纳米铜。
2.3 催化实验称取不同质量催化剂加入30 mL 0.15 mmol·L-1 RhB溶液中超声分散溶解,再缓慢加入10 mL新配制NaBH4溶液(25 mmol·L-1),采用紫外-可见分光光度计检测溶液在200~800 nm波长吸光度随时间的变化。
3 实验结果与讨论 3.1 Cu@rGO纳米复合材料表征图 1分别为不同rGO/Cu比例Cu@rGO纳米复合材料的TEM图。如图所示,原料配比rGO:Cu=1:1、1:3、1:5的纳米复合材料样品的平均粒径分别为5.69 nm,2.10 nm和6.56 nm,原料配比rGO:Cu=1:1和1:5的纳米颗粒粒径分布较宽(2.5~10.5 nm和3.5~11.5 nm),且存在部分团聚现象,原料配比rGO:Cu=1:3的纳米颗粒粒径分布较窄(1.5~2.5 nm),具有良好的分散性。
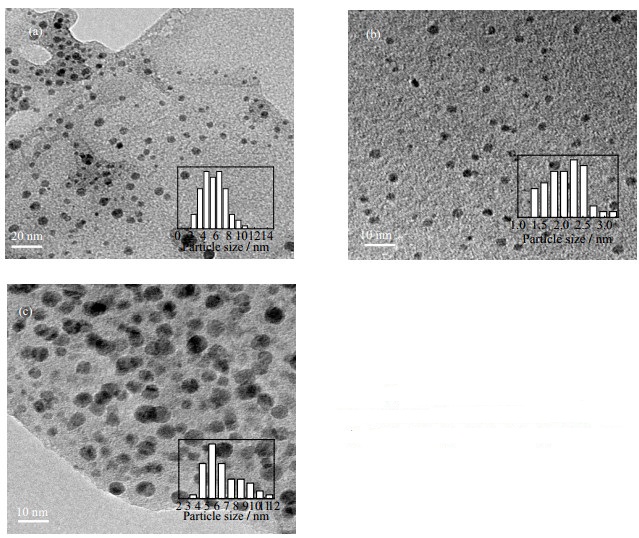
|
图 1 Cu@rGO复合材料的TEM图 Fig.1 TEM micrographs of the Cu@rGO nanocomposites (a) rGO:Cu=1:1 (b) rGO:Cu=1:3 (c) rGO:Cu=1:5 |
Cu@rGO纳米复合材料的XRD图谱如图 2所示。结果显示,样品在2θ为43.3°,50.4°和74.3°处有明显的衍射峰,对应立方晶系铜(111)、(200)和(220)晶面(No.04-0836)。rGO:Cu=1:1的样品XRD图谱中还存在36.6°的衍射峰,表明样品中还存在部分Cu2O(No.05-0667)。在Cu@rGO纳米复合材料XRD图谱中,2θ为11.0°,24.0°左右没有出现GO和rGO的衍射峰,说明GO被还原形成了rGO,在复合材料的制备过程中,引入rGO的含量较低,Cu颗粒的存在有效抑制了rGO片层的有序堆叠。
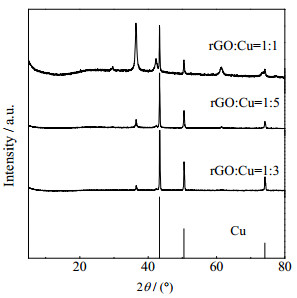
|
图 2 Cu@rGO纳米复合材料的XRD图谱 Fig.2 XRD patterns of Cu@rGO nanocomposites |
GO与Cu@rGO纳米复合材料的红外光谱如图 3所示,在1 050 cm-1处的吸收峰对应环氧基中C-O的伸缩振动,在1 425 cm-1处的吸收峰对应C─OH官能团中C─O的弯曲振动,在1 610 cm-1处的吸收峰对应O─H的弯曲振动,1 720 cm-1出现了C═O的伸缩振动吸收峰,在3 210和3 380 cm-1处的吸收峰分别对应═C─H和─OH的伸缩振动。而Cu@rGO纳米复合材料中含氧官能团明显降低或消失,表明在Cu@rGO纳米复合材料的制备过程中,GO被有效地还原为rGO。
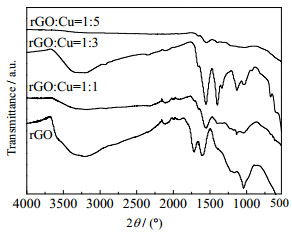
|
图 3 GO与Cu@rGO纳米复合材料的FTIR光谱图 Fig.3 FTIR spectra of GO and Cu@rGO nanocomposites |
3种原料配比的Cu@rGO纳米复合材料的XPS图给出了Cu@rGO纳米复合材料中不同价态铜的结合能(图 4),Cu 2p3/2的结合能为933.45 eV,Cu 2p1/2的结合能为953.95 eV,表明Cu@rGO复合材料中铜主要以单质铜的形式存在。
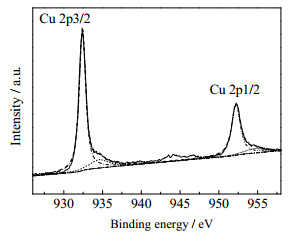
|
图 4 Cu@rGO复合材料的XPS图谱 Fig.4 XPS patterns of Cu@rGO nanocomposites |
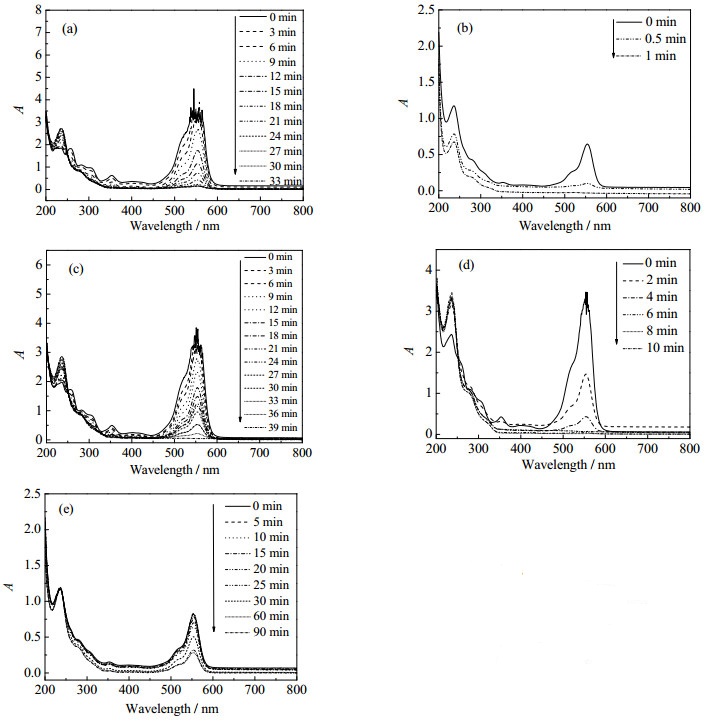
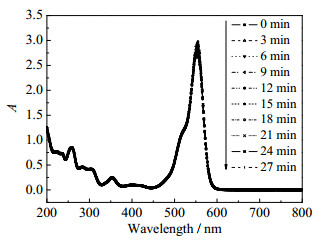
图 5为以NaBH4做还原剂降解RhB的紫外-可见吸收光谱图。其中图 5(a)~5(d)分别为NaBH4存在下,不同rGO/Cu比例的Cu@rGO纳米复合材料及纯铜催化降解RhB的紫外-可见吸收曲线随降解时间的变化(催化剂用量为0.001 g)。可以看出,当rGO:Cu=1:3时,554 nm处吸光度值下降最快,也即溶液褪色最为迅速,染料降解所需时间远小于纯铜与其他比例催化剂体系,表明原料配比rGO:Cu=1:3的Cu@rGO纳米复合材料对RhB降解的催化效果最显著。图 5(e)为不添加Cu@rGO纳米复合材料催化时溶液体系吸收曲线随降解时间的变化,可以看出随着时间增加,吸光度值逐渐降低,但下降趋势缓慢,90 min仍未降解完全,表明在没有纳米复合材料催化辅助时,还原过程进行得非常缓慢。这可能是由于催化剂中石墨烯具有较大比表面积,可提供更多的活性位点且具有更高的电子迁移率,且rGO:Cu=1:3的Cu@rGO纳米复合材料负载的Cu粒径相对较小,分散性好,因而该原料配比的催化剂催化NaBH4还原RhB效果最佳。图 6为不添加还原剂NaBH4时RhB降解的吸收曲线,由图可以看出只添加Cu@rGO复合材料时,随着反应时间的增加,27 min内吸收曲线基本重合,吸光度值几乎无变化,表明Cu@rGO复合材料对RhB无吸附作用。
|
图 5 RhB降解的紫外-可见吸收光谱图 Fig.5 UV-Vis absorption spectra for RhB degradation with NaBH4 (a) rGO:Cu=1:1 (b) rGO:Cu=1:3 (c) rGO:Cu=1:5 (d) Cu (e) without catalyst |
|
图 6 RhB降解的紫外-可见吸收光谱图(无NaBH4) Fig.6 UV-Vis absorption spectra for RhB degradation without NaBH4 |
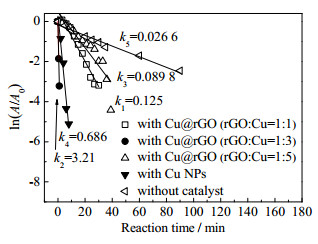
跟踪染料降解过程中反应体系吸光度变化,采用一级动力学描述RhB降解反应过程[34-36],催化反应过程中NaBH4过量,反应速率不受NaBH4浓度影响,可采用ln(A/A0)=-kt描述催化反应动力学,其中k为表观一级动力学常数(min-1),t为反应时间(min),A0和A分别表示染料在初始和时间t时的吸光度[37],与相应时间的染料浓度成正比。ln(A/A0)与反应时间t的线性关系如图 7所示。由图可见,ln(A/A0)与反应时间t线性关系良好,表观反应速率常数k可由各自线性方程得到。仅加入NaBH4时RhB降解速率较小,k=0.0266 min-1,加入含Cu催化剂后RhB降解速率均有明显增加,反映了Cu催化剂具有催化效果,其中原料配比rGO:Cu=1:3的Cu@rGO复合材料对降解反应的催化效果最明显,表观反应速率常数(k2=3.21 min-1)约为Cu颗粒催化体系反应速率常数(k4=0.686 min-1)的5倍。反应速率常数增加直接反映了RhB染料降解效率增加,表明rGO:Cu=1:3的Cu@rGO纳米复合材料对染料降解反应具有良好的催化活性。
|
图 7 ln(A/A0)与反应时间的线形关系图 Fig.7 Plots of ln(A/A0) against reaction time t for RhB degradation |
表 1比较了Cu@rGO纳米复合材料(rGO:Cu =1:3)与其他已报道催化剂催化NaBH4还原RhB的反应时间。由表 1可见,还原相同量RhB时,本实验制备的催化剂完成降解反应所需时间最短,且催化剂用量最少。这可能是由于纳米Cu颗粒与rGO协同作用增加了电子转移速率,进而提高了催化反应速率。
|
|
表 1 不同催化剂条件下NaBH4还原RhB所需时间 Table 1 Completion time for the reduction of RhB by different catalysts in the presence of NaBH4 |
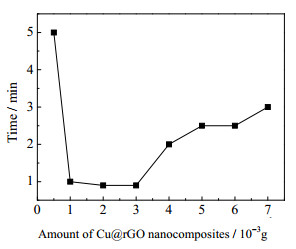
进一步考察Cu@rGO纳米复合材料用量对RhB催化效果的影响,选用rGO:Cu=1:3的Cu@rGO纳米复合材料,控制催化剂用量分别为0.000 5~0.007 g,其它条件不变,RhB溶液完全褪色所需时间随催化剂浓度的变化关系如图 8所示。可以看出,RhB降解反应完成时间随着催化剂用量的增加有先减小后增加的趋势,完全反应所需最短时间小于1 min,随后反应时间略有上升,这可能是由于催化剂用量0.003 g时产生具有效催化活性的分子已接近最大值,继续增加催化剂用量不利于促进更多活性物质产生,且rGO的过多引入可能降低催化剂有效浓度,导致催化反应时间增加,催化效果降低。考虑到催化剂添加量在0.001 ~0.003 g时反应时间并无明显下降,从经济性的角度考虑,添加量控制在0.001 g左右即可。
|
图 8 Cu@rGO(rGO:Cu=1:3)纳米复合材料用量对RhB催化降解效果的影响 Fig.8 Effects of Cu@rGO nanocomposite (rGO:Cu=1:3) amounts on time required for the completion of RhB degradation |
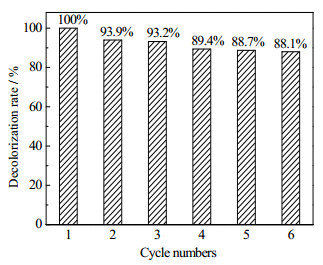
无负载的铜纳米粒子在参与催化反应过程中易团聚,且不易分离,阻碍其循环使用。本论文考察了rGO:Cu =1:3的Cu@rGO复合材料的稳定性,在RhB降解反应结束后,将Cu@rGO纳米复合材料离心分离,再重新进行催化实验,重复6次结果见图 9。Cu@rGO复合材料在经过6次循环使用后,催化活性没有明显的变化,对RhB的催化降解率仍然在88.0%以上,表明其具有较好的循环稳定性。这可能是由于石墨烯的存在能有效的减少Cu纳米颗粒的团聚,并提升了Cu纳米颗粒的抗氧化能力。
|
图 9 Cu@rGO(rGO:Cu=1:3)纳米复合材料催化降解RhB的稳定性 Fig.9 Stability results of Cu@rGO nanocomposites (rGO:Cu=1:3) for catalytic RhB degradation |
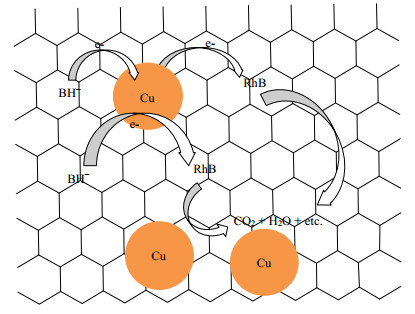
关于金属纳米粒子催化氧化还原反应已经有广泛的研究[44-45]。一般认为,催化还原反应发生在金属纳米粒子表面,反应物富集于催化剂表面,NaBH4首先与金属纳米粒子反应生成金属氢化物,进而吸附在催化剂表面的有机染料分子得到氢被还原[45]。从热力学的角度上,催化还原反应的活化能取决于催化剂的表面,催化剂粒径越小,其表面越粗糙,相应具有更高的活性,使得催化还原反应的活化能更低[46]。催化RhB反应机理的简单示意图示于图 10。BH4-还原RhB的主要产物为CO2、H2O及其它有机分子[2]。Cu@rGO纳米复合材料催化还原染料是电子转移的过程,催化剂表面的硼氢化钠作为亲核试剂可提供e-给纳米Cu,含有π-π键的染料分子作为电子受体捕获来自纳米Cu的电子,即BH4-通过纳米Cu将e-转移至染料分子,反应中间体从生成的氢化物得到氢,在金属催化剂表面发生电子转移诱导的加氢还原反应,反应完成后还原产物最终从催化剂表面脱附[7]。由于Cu@rGO纳米复合材料中纳米铜具有极小的粒径和较大的比表面积,因此具有极高的催化活性,且rGO载体的协同作用可有效的提高电子迁移能力,同时阻止纳米Cu颗粒的团聚,提高纳米Cu颗粒的稳定性,使更多反应物分子接触纳米Cu表面,提供了更多的反应活性位点,反应物分子在催化剂表面的富集提高了局部反应物浓度,进一步提高了纳米复合材料的催化性能。
|
图 10 Cu@rGO(rGO:Cu=1:3)纳米复合材料催化降解染料机理 Fig.10 Mechanism of catalytic reduction and degradation of dyes by Cu@rGO nanocomposites (rGO:Cu=1:3) |
以功能化的石墨烯为模板负载铜纳米粒子,制备不同原料配比的Cu@rGO纳米复合材料,研究了不同原料配比对纳米复合材料的形貌和粒径的影响,以罗丹明B为模拟污染物考察了其催化降解性能。原料配比rGO:Cu=1:3时,制备的Cu@rGO复合材料中纳米铜呈类球型颗粒,纳米尺寸极小(2.10 nm)且粒径分布窄。染料降解实验表明rGO:Cu=1:3的纳米复合材料对RhB的催化降解效果最好,优于多数文献报道的催化剂。且经过6次循环使用后,催化剂活性无明显变化,具有良好的稳定性。结果表明Cu@rGO纳米复合材料制备方法简单且催化效果较好,具有应用价值。
|
|
| [1] |
WANG Y, HE Y, LAI Q, et al. Review of the progress in preparing nano TiO2: An important environmental engineering material[J]. Journal of Environmental Sciences (China), 2014, 26(11): 2139-2177. DOI:10.1016/j.jes.2014.09.023 |
| [2] |
ZHANG Y, ZHU P, CHEN L, et al. Hierarchical architectures of monodisperse porous Cu microspheres: synthesis, growth mechanism, high-efficiency and recyclable catalytic performance[J]. Journal of Materials Chemistry A, 2014, 2(30): 11966-11973. DOI:10.1039/C4TA01920B |
| [3] |
MARIEN C B D, COTTINEAU T, ROBERT D, et al. TiO2 nanotube arrays: Influence of tube length on the photocatalytic degradation of paraquat[J]. Applied Catalysis B: Environmental, 2016, 194: 1-6. DOI:10.1016/j.apcatb.2016.04.040 |
| [4] |
VIDHU V K, PHILIP D. Catalytic degradation of organic dyes using biosynthesized silver nanoparticles[J]. Micron, 2014, 56: 54-62. DOI:10.1016/j.micron.2013.10.006 |
| [5] |
AKSU Z. Application of biosorption for the removal of organic pollutants: a review[J]. Process Biochemistry, 2005, 40: 997-1026. DOI:10.1016/j.procbio.2004.04.008 |
| [6] |
NEUS G B, FLORIND M, JORDI P, et al. Synthesis of highly monodisperse citrate-stabilized silver nanoparticles of up to 200 nm: Kinetic control and catalytic properties[J]. Chemsity of Materials, 2014, 26(9): 2836-2846. DOI:10.1021/cm500316k |
| [7] |
MONIREH A, MAHMOUD N, MOHAMMAD S. Green synthesis of a Cu/reduced graphene oxide/Fe3O4 nanocomposite using euphorbia wallichii leaf extract and its application as a recyclable and heterogeneous catalyst for the reduction of 4-nitrophenol and Rhodamine B[J]. RSC Advances, 2015, 5(111): 91532-91543. DOI:10.1039/C5RA17269A |
| [8] |
HU Y, YANG X, CAO S, et al. Effect of the dispersants on Pd species and catalytic activity of supported palladium catalyst[J]. Applied Surface Science, 2017, 400: 148-153. DOI:10.1016/j.apsusc.2016.12.182 |
| [9] |
ZOU C, YANG B, BIN D, et al. Electrochemical synthesis of gold nanoparticles decorated flower-like graphene for high sensitivity detection of nitrite[J]. Journal of Colloid and Interface Science, 2017, 488: 135-141. DOI:10.1016/j.jcis.2016.10.088 |
| [10] |
李娟, 赵安婷, 邵姣婧, 等. 铜/石墨烯复合材料的制备及催化性能[J]. 无机化学学报, 2017, 33(7): 1231-1235. LI J, ZHAO A T, SHAO J J, et al. Preparation and catalytic properties of copper/graphene composites[J]. Chinese Journal of Inorganic Chemistry, 2017, 33(7): 1231-1235. |
| [11] |
雷彬, 张旭, 谭文松, 等. 生物纳米钯催化剂的制备、表征及其合成机理初探[J]. 高校化学工程学报, 2015, 29(4): 873-880. LEI B, ZHANG X, TAN W S, et al. Preparation, characterization and synthesis mechanism of biopalladium nanoparticles produced by klebsiella pneumoniae ECU-15[J]. Journal of Chemical Engineering of Chinese Universities, 2015, 29(4): 873-880. DOI:10.3969/j.issn.1003-9015.2015.04.015 |
| [12] |
SUN L, WANG G, HAO R, et al. Solvothermal fabrication and enhanced visible light photocatalytic activity of Cu2O-reduced graphene oxide composite microspheres for photodegradation of Rhodamine B[J]. Applied Surface Science, 2015, 358: 91-99. DOI:10.1016/j.apsusc.2015.08.128 |
| [13] |
SARDAR R, BEASLEY C A, MURRAY R W. Interfacial ion transfers between a monolayer phase of cationic Au nanoparticles and contacting organic solvent[J]. Journal of the American Chemical Society, 2010, 132(6): 2058-2063. DOI:10.1021/ja909584p |
| [14] |
ALMEIDA B M, MELO M A, BETTINI J, et al. A novel nanocomposite based on TiO2/Cu2O/reduced graphene oxide with enhanced solar-light-driven photocatalytic activity[J]. Applied Surface Science, 2015, 324: 419-431. DOI:10.1016/j.apsusc.2014.10.105 |
| [15] |
LI H, SU Z, HU S, et al. Free-standing and flexible Cu/Cu2O/CuO heterojunction net: A novel material as cost-effective and easily recycled visible-light photocatalyst[J]. Applied Catalysis B: Environmental, 2017, 207: 134-142. DOI:10.1016/j.apcatb.2017.02.013 |
| [16] |
CHOI J, OH H, HAN S W, et al. Preparation and characterization of graphene oxide supported Cu, Cu2O, and CuO nanocomposites and their high photocatalytic activity for organic dye molecule[J]. Current Applied Physics, 2017, 17(2): 137-145. DOI:10.1016/j.cap.2016.11.020 |
| [17] |
XU H, FENG J X, TONG Y X, et al. Cu2O-Cu hybrid foams as high-performance electrocatalysts for oxygen evolution reaction in alkaline media[J]. Acs Catalysis, 2016, 7(2): 986-991. |
| [18] |
WANG X, HUANG Y, CHEN Y. Focusing on energy and optoelectronic applications: a journey for graphene and graphene oxide at large scale[J]. Accounts of Chemical Research, 2012, 45(4): 598-607. DOI:10.1021/ar200229q |
| [19] |
STANKOVICH S, DIKIN D A, DOMMETT G H B, et al. Graphene-based composite materials[J]. Nature, 2006, 442: 282-286. DOI:10.1038/nature04969 |
| [20] |
NOVOSELOV K S, GEIM A K, MOROZOV S V, et al. Electric field effect in atomically thin carbon films[J]. Science, 2004, 306: 666-669. DOI:10.1126/science.1102896 |
| [21] |
XIANG Q, YU J, JARONIEC M. Graphene-based semiconductor photocatalysts[J]. Chemicl Society Reviews, 2012, 41(2): 782-796. DOI:10.1039/C1CS15172J |
| [22] |
HUANG X, QI X Y, BOEY F, et al. Graphene-based composites[J]. Chemical Society Reviews, 2012, 41(2): 666-686. DOI:10.1039/C1CS15078B |
| [23] |
陈子潇, 沈静, 徐然, 等. 可磁回收Cu-Fe3O4@GE复合材料催化还原对硝基苯酚的研究[J]. 高校化学工程学报, 2018, 32(3): 577-585. CHEN Z X, SHEN J, XU R, et al. Catalytic reduction of p-nitrophenol with magnetic Cu-Fe3O4@GE composites[J]. Journal of Chemical Engineering of Chinese Universities, 2018, 32(3): 577-585. DOI:10.3969/j.issn.1003-9015.2018.03.012 |
| [24] |
徐超, 陈胜, 汪信. 基于石墨烯的材料化学进展[J]. 应用化学, 2011, 28(1): 1-9. XU C, CHEN S, WANG X. Progress in the chemistry of materials based on graphene[J]. Chinese Journal of Applied Chemistry, 2011, 28(1): 1-9. DOI:10.3969/j.issn.1001-4160.2011.01.001 |
| [25] |
GEIM A K. Graphene: status and prospects[J]. Science, 2009, 324: 1530-1534. DOI:10.1126/science.1158877 |
| [26] |
YANG Y, TIAN C, WANG J, et al. Facile synthesis of novel 3D nanoflower-like Cu(x)O/multilayer graphene composites for room temperature NO(x) gas sensor application[J]. Nanoscale, 2014, 6(13): 7369-7378. DOI:10.1039/c4nr00196f |
| [27] |
毕慧平, 刘立忠, 丁佳佳, 等. Cu-石墨烯Fenton催化剂的制备及催化活性[J]. 无机化学学报, 2014, 30(10): 2347-2352. BI H P, LIU L Z, DING J J, et al. Preparation and catalytic property of Cu-graphene fenton-like catalyst[J]. Chinese Journal of Inorganic Chemistry, 2014, 30(10): 2347-2352. |
| [28] |
LIU S, TIAN J Q, WANG L, et al. One-pot synthesis of CuO nanoflower-decorated reduced graphene oxide and its application to photocatalytic degradation of dyes[J]. Catalysis Science & Technology, 2012, 2(2): 339-344. |
| [29] |
ZHU M, ZHAI C, SUN M, et al. Ultrathin graphitic C3N4 nanosheet as a promising visible-light-activated support for boosting photoelectrocatalytic methanol oxidation[J]. Applied Catalysis B: Environmental, 2017, 203: 108-115. DOI:10.1016/j.apcatb.2016.10.012 |
| [30] |
SINGH H P, GUPTA N, SHARMA S K, et al. Synthesis of bimetallic Pt-Cu nanoparticles and their application in the reduction of rhodamine B[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2013, 416: 43-50. |
| [31] |
LI X, WANG X, SONG S Y, et al. Selectively deposited noble metal nanoparticles on Fe3O4/graphene composites: Stable, recyclable, and magnetically separable catalysts[J]. Chemistry-A European Journal, 2012, 18(24): 7601-7607. DOI:10.1002/chem.201103726 |
| [32] |
JR W S H, OFFEMAN R E. Preparation of graphitic oxide[J]. Journal of the American Chemical Society, 1958, 80(6): 1339. DOI:10.1021/ja01539a017 |
| [33] |
HUANG S Y, ZHANG K, YUEN M F, et al. Facile synthesis of flexible graphene-silver composite papers with promising electrical and thermal conductivity performances[J]. Rsc Advances, 2014, 4(64): 34156-34160. DOI:10.1039/C4RA05176A |
| [34] |
AI L, YUE H, JiANG J. Environmentally friendly light-driven synthesis of Ag nanoparticles in situ grown on magnetically separable biohydrogels as highly active and recyclable catalysts for 4-nitrophenol reduction[J]. Journal of Materials Chemistry, 2012, 22(44): 23447-23453. DOI:10.1039/c2jm35616c |
| [35] |
GHOSH S, PATIL S, AHIRE M, et al. Gnidia glauca flower extract mediated synthesis of gold nanoparticles and evaluation of its chemocatalytic potential[J]. Journal of nanobiotechnology, 2012, 10: 17. DOI:10.1186/1477-3155-10-17 |
| [36] |
ZHANG Z, SHANG C, SUN Y, et al. Tubular nanocomposite catalysts based on size-controlled and highly dispersed silver nanoparticles assembled on electrospun silica nanotubes for catalytic reduction of 4-nitrophenol[J]. Journal of Materials Chemistry, 2011, 22(4): 1387-1395. |
| [37] |
GAO S, JIA X, YANG J, et al. Hierarchically micro/nanostructured porous metallic copper: Convenient growth and superhydrophilic and catalytic performance[J]. Journal of Materials Chemistry, 2012, 22(40): 21733-21739. DOI:10.1039/c2jm35233h |
| [38] |
YANG X, ZHONG H, ZHU Y, et al. Highly efficient reusable catalyst based on silicon nanowire arrays decorated with copper nanoparticles[J]. Journal of Materials Chemistry A, 2014, 2(24): 9040-9047. DOI:10.1039/c4ta00119b |
| [39] |
ZHANG B, ZHAO B, HUANG S, et al. One-pot interfacial synthesis of Au nanoparticles and Au-polyaniline nanocomposites for catalytic applications[J]. CrystEngComm, 2012, 14(5): 1542-1544. DOI:10.1039/c2ce06396d |
| [40] |
AI L, ZENG C, WANG Q. One-step solvothermal synthesis of Ag-Fe3O4 composite as a magnetically recyclable catalyst for reduction of rhodamine B[J]. Catalysis Communications, 2011, 14(1): 68-73. DOI:10.1016/j.catcom.2011.07.014 |
| [41] |
JIANG Z J, LIU C Y, SUN L W. Catalytic properties of silver nanoparticles supported on silica spheres[J]. Journal of Physical Chemistry B, 2005, 109(5): 1730-1735. DOI:10.1021/jp046032g |
| [42] |
DENG Z, ZHU H, PENG B, et al. Synthesis of PS/Ag nanocomposite spheres with catalytic and antibacterial activities[J]. ACS Applied Materials & Interfaces, 2012, 4(10): 5625-5632. |
| [43] |
GHOSH B K, HAZRA S, NAIK B, et al. Preparation of Cu nanoparticle loaded SBA-15 and their excellent catalytic activity in reduction of variety of dyes[J]. Powder Technology, 2015, 269: 371-378. DOI:10.1016/j.powtec.2014.09.027 |
| [44] |
PABLO H, MOISES P, LUIS M L, et al. Catalysis by metallic nanoparticles in aqueous solution: Model reactionsw[J]. Chemical Society Reviews, 2012, 41(17): 5577-5587. DOI:10.1039/c2cs35029g |
| [45] |
STEFANIE W, FRANK P, YAN L, et al. Kinetic analysis of catalytic reduction of 4-nitrophenol by metallic nanoparticles immobilized in spherical polyelectrolyte brushes[J]. Journal of Physical Chemistry C, 2010, 114(19): 8814-8820. DOI:10.1021/jp101125j |
| [46] |
PANIGRAHI S, BASU S, PRAHARAJ S, et al. Synthesis and size-selective catalysis by supported gold nanoparticles: Study on heterogeneous and homogeneous catalytic process[J]. Journal of Physical Chemistry C, 2007, 111(12): 4596-4605. DOI:10.1021/jp067554u |




