2. 洞庭湖水环境治理与生态修复湖南省重点实验室, 湖南 长沙 410114;
3. 河南工业大学环境工程学院, 河南 郑州 450001
2. Key Laboratory of Dongting Lake Aquatic Eco-Environmental Control and Restoration of Hunan Province, Changsha 410114, China;
3. College of Environmental Engineering, Henan University of Technology, Zhengzhou 450001, China
膜分离作为21世纪新型的水处理技术受到了广泛的关注。2021年我国膜产业已形成3 000亿规模、“十四五”规划的万亿发展远景[1]。然而,目前膜法水处理技术仍受到选择性差、膜污染严重等问题的限制,新型膜材料的开发有望解决这些问题,为进一步拓展膜法水处理技术的应用奠定基础[2]。由金属离子和有机配体组成的金属有机框架材料(MOFs)具有多孔、大表面积和易于功能化等特点,是近几年最受关注的膜改性材料之一[3-4]。其中,Zr-MOFs在水处理领域的应用最广泛[5]。
根据软硬酸碱理论,由硬酸Zr4+和羧酸配体构成的Zr-MOFs含有强配位键,具有优异的化学和水热稳定性,可以适应复杂多变的进水水质[6]。同时,Zr-MOFs的配体含有多种官能团,易于通过修饰改变表面性质,一方面,可以获得结构良好和性质优异的Zr-MOFs复合膜,另一方面,赋予Zr-MOFs复合膜多种功能[7-8],对水中难降解污染物的去除有重要意义。因此,与传统滤膜相比,Zr-MOFs复合膜具有更高的亲水性、抗污染性和选择分离性,在水处理中有广阔的应用前景。
近年来,Zr-MOFs复合膜被大量开发,在Zr-MOFs复合膜制备策略的研究中取得了突破性进展,制得的高性能Zr-MOFs复合膜在水处理领域有广泛应用[9-14]。本文综述了Zr-MOFs复合膜的成膜方法、基本性质和水处理效能的研究进展,重点分析了Zr-MOFs复合膜成膜方法的优化策略及其对各种污染物的去除机制,最后对Zr-MOFs复合膜在水处理中的应用优势及面临的挑战进行了总结和展望。
2 Zr-MOFs材料水处理膜改性制备中常用的Zr-MOFs包括UiO-66(University of Oslo)系列[15-19]、PCN(Porous Coordination Network)系列[11, 13, 20-22]、MOF-801[12]和MOF-808[23],它们的孔径和比表面积如表 1所示。
|
|
表 1 典型Zr-MOFs的结构性质参数 Table 1 Structural and property parameters of typical Zr-MOFs |
由Zr4+与羧酸配体配位而成的UiO-66及配体功能化的UiO-66是目前水处理膜的改性制备中应用最广泛的Zr-MOFs[15]。UiO-66在pH为1~11的溶液中可长期保持稳定,结构中的苯环碳与羧酸碳的键在540 ℃下才断裂[16]。通过将氨基、羟基和羧基等功能基团引入UiO-66中,可以改变UiO-66的表面性质和结构可调性,同时增强对特定污染物的亲和力[17]。最常见的是UiO-66-NH2。由于氨基的存在,UiO-66-NH2的亲水性更强、更易进行修饰和改性。UiO-66-(COOH)2含有24个游离的羧酸基团,对稀土金属元素有较强的亲和力[18],而UiO-66-(OH)2的孔径尺寸仅为0.4 nm,有利于单价盐离子的截留[19]。
2.2 PCN系列由Zr4+与卟啉配体构建而成的PCN-222和PCN-224在水处理膜的制备中也有应用[11, 13]。PCN-222在酸性溶液中稳定[20],在稀碱溶液中易发生取代反应而分解[21]。PCN-224在酸性及弱碱性溶液中均有较高的稳定性[22]。总体上,PCN系列材料的比表面积大于UiO-66系列,而且具有多种功能的卟啉配体和配位不饱和Zr4+使其易于功能化修饰。
2.3 MOF-801和MOF-808由Zr4+与富马酸配体构建而成MOF-801,具有极强的吸水性,与UiO-66的拓扑结构相同,但是孔径尺寸略小于UiO-66[12]。MOF-808是由Zr金属簇与均苯三甲酸配位而成的孔径较大的Zr-MOFs[23],结构中的多个官能团为有机物和重金属等多种污染物提供了丰富的吸附位点。
3 Zr-MOFs复合膜的制备方法 3.1 共混法共混法是制备Zr-MOFs/聚合物复合膜常用的方法之一。在共混过程中,Zr-MOFs和聚合物在有机溶剂中混合均匀,然后通过相转化或静电纺丝法形成混合基质膜[9-10, 14, 24-25]。由于Zr-MOFs的有机配体与聚合物之间有较好的相容性,Zr-MOFs混合基质膜呈现出良好的膜结构和分离性能。然而,Zr-MOFs含量过高时易团聚,且结构中的金属离子与聚合物之间相容性较差,导致复合膜表面产生缺陷。Yin等[25]发现,当UiO-66的负载量(质量分数)为20% 时,复合膜中UiO-66均匀分布在聚偏氟乙烯(PVDF)基质中。然而,当UiO-66的负载量增加到30% 时,复合膜中出现了明显的颗粒团聚。
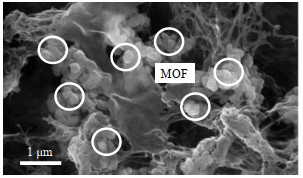
如何获得无缺陷的Zr-MOFs混合基质膜是研究人员关注的重点问题。为了避免Zr-MOFs的团聚,El-Mehalmey等[14]利用新合成的湿MOFs晶体构建UiO-66-NH2@醋酸纤维素(CA)混合基质膜,从该膜的扫描电镜(SEM)图可以看出(见图 1),UiO-66-NH2纳米粒子均匀分布在CA基质中。然而,干湿MOFs含有相同的化学基团,Zr-MOFs与聚合物之间的相互作用并未改变,对Zr-MOFs或聚合物进行修饰则可促进二者之间的相互作用[26-28]。研究显示,经聚甲基丙烯酸磺基丁酯(PSBMA)改性的UiO-66-NH2在铸膜液中表现出更好的分散性和相容性,这是由于PSBMA上的疏水链与聚砜(PSf)之间的相互作用提升了二者之间的相容性[26]。Al-Shaeli等[27]发现,氯磺酸磺化的聚醚砜(PES)与UiO-66和UiO-66-NH2有良好的相容性。这是因为氯磺酸不仅可以与UiO-66和UiO-66-NH2中的─NH2和─COOH反应,还可以作为偶联剂将PES和MOFs连接在一起。
通过Zr-MOFs或聚合物的改性,两者之间的相容性显著提升,Zr-MOFs复合膜界面缺陷的形成得到有效控制。但是,目前关于Zr-MOFs与聚合物之间相互作用机制的研究还比较缺乏,后续可以深入解析两者之间的相互作用机制,为进一步优化Zr-MOFs复合膜的共混法制备提供理论指导。
3.2 界面聚合法UiO-66、UiO-66-NH2、MOF-801和PCN-222等Zr-MOFs可以通过分散在水相或有机相中参与界面聚合反应,掺入由PES、聚丙烯腈(PAN)、PSf等聚合物层支撑的聚酰胺(PA)层中,制备得到Zr-MOFs改性的薄膜纳米复合材料(TFN)膜[12-13, 15, 29-34]。
Zr-MOFs的负载量对PA层的形态结构影响显著。当负载量较低时,PCN-222和UiO-66主要存在于PA层内,TFN膜的表面形态并未发生显著改变;当负载量较高时,Zr-MOFs在TFN膜表面发生局部聚集而导致膜面产生缺陷[13, 35]。此外,Zr-MOFs的引入途径会对PA层的厚度产生影响。Zhang等[15]将UiO-66和UiO-66-NH2分别分散在含有均苯三甲酰氯(TMC)的有机相和含有聚乙烯亚胺(PEI)和哌嗪(PIP)的水相中,通过界面聚合法制备得到Zr-MOFs-TFN膜,其中UiO-66改性的TFN膜的厚度最大(69.7 nm),这是因为UiO-66分散在有机相中,对水相中PIP和PEI分子向有机相中的扩散没有产生显著影响[15]。综上所述,Zr-MOFs引入后,PA层的形貌和厚度等结构参数发生变化,优化界面聚合法以获得无缺陷的Zr-MOFs-TFN膜至关重要。
目前,促进Zr-MOFs在溶液相中的分散,提高其与PA层的相容性是减少TFN膜界面缺陷的重要途径。研究显示,使用预干燥的Zr-MOFs[29, 36]、将含有Zr-MOFs的溶液在冰水中进行超声处理[37-38]或对Zr-MOFs进行改性[30]都可以实现Zr-MOFs的均匀分散。通过压力辅助或聚合物改性等方式增强Zr-MOFs与多孔基质之间的相互作用,也可以有效抑制界面聚合反应过程中粒子的团聚[12, 39]。此外,部分Zr-MOFs中的氨基可以促进其与TMC中酰氯键的共价结合,显著减少Zr-MOFs-TFN膜的界面缺陷[29, 39]。
3.3 原位生长法原位生长主要用于Zr-MOFs无机复合膜的制备中[19, 40-42]。目前,可以通过水热合成或二次生长在Al2O3多孔基质或不锈钢网上制备得到含有UiO-66、UiO-66-NH2和UiO-66-(OH)2的Zr-MOFs复合膜。由于Zr-MOFs和支撑层之间的结合力较弱,很难在多孔基质表面形成连续和致密的功能层。Zhu等[42]将多巴胺预涂在不锈钢网上,增强了原位生长过程中Cu-UiO-66-NH2与不锈钢网之间的结合力。Wang等[19]通过二次生长法修复UiO-66-(OH)2/Al2O3膜的表面缺陷,缓解了多晶UiO-66-(OH)2膜中的配体缺失。
3.4 压力协助过滤法研究者采用压力协助过滤法,将UiO-66、UiO-66-NH2、PCN-224等Zr-MOFs沉积在PES和PVDF等微滤膜上,制备得到复合膜[11, 43-50]。制膜过程中,添加聚丙烯酸(PAA)可以提高Zr-MOFs的亲水性及其在水中的分散性[46-48]。此外,还应着重考虑复合膜的稳定性。Zhang等[45]利用交联剂PEI促进功能层与支撑层之间的相互作用,提高UiO-66-NH2/PVDF复合膜的稳定性。Liu等[43]在铸膜液中添加聚多巴胺(PDA),通过PDA与UiO-66之间的强结合力显著提升了复合膜的稳定性。
4 Zr-MOFs复合膜的特性 4.1 表面特性 4.1.1 亲疏水性Zr-MOFs复合膜的亲疏水性是Zr-MOFs膜在水处理中应用的重要特性。较强的亲水性可以促进水分子渗透到膜内,获得较高的纯水通量。亲水性强的Zr-MOFs改性后的复合膜亲水性显著提升。引入具有较多亲水性基团(─NH和─NH2等)的Zr-MOFs可以进一步提高复合膜的亲水性[29]。Zr-MOFs的负载量对复合膜亲水性的影响需要关注。研究显示,随着Zr-MOFs负载量的增加,膜的表面亲水性呈增加趋势[26, 51-52]。然而,当Zr-MOFs过量时,膜的亲水性出现下降趋势。这是因为过量的Zr-MOFs会堵塞膜的内部通道,增加亲水组分向膜表面迁移的阻力,进而减少膜面亲水基团的数量,导致亲水性下降[43]。
4.1.2 粗糙度表面粗糙度对膜的抗污染性能影响显著。通常,粗糙度低的膜具有较好的抗污染性能[53-54]。适量的Zr-MOFs可以填充在膜表面的褶皱结构中而使复合膜的粗糙度降低。然而,当Zr-MOFs过量时,它们在膜表面的聚集使膜的粗糙度再次增加[31]。因此,在Zr-MOFs复合膜的制备过程中,控制Zr-MOFs的负载量对于复合膜粗糙度的调控非常关键。
4.1.3 Zeta电位膜的表面电荷是影响膜分离性能的重要因素[55-56]。Zr-MOFs对复合膜电性的影响可以从两方面解释。首先,Zr-MOFs中的带电基团使膜的表面电荷受到影响。Liu等[51]将带正电的UiO-66-NH2引入PA层后,TFN膜呈正电性,复合膜的Zeta电位值随着UiO-66-NH2负载量的增加而增加。其次,Zr-MOFs会对成膜过程发生的化学反应产生影响,最终使复合膜的表面电荷发生变化。例如,UiO-66-NH2-TFN膜的Zeta电位值高于原始膜,而UiO-66-TFN膜的Zeta电位值低于原始膜[15]。这是因为,在界面聚合的过程中,水溶液中的UiO-66-NH2阻止PIP和PEI单体迁移到有机相,导致复合膜中的─COOH含量降低而使UiO-66-NH2-TFN膜的Zeta电位值增高。对于UiO-66-TFN膜,有机溶液中的UiO-66会阻碍TMC参与界面聚合反应,未反应的酰氯基团导致复合膜的电荷密度低于原始膜[15]。
4.2 水渗透性膜的水渗透性是评估膜性能的重要参数。研究显示,Zr-MOFs复合膜的水渗透性远大于原始膜[50]。Ma等[57]测试了PES膜,氧化石墨烯(GO)改性的PES膜和UiO-66@GO/PES膜的纯水通量,与PES膜和PES/GO膜相比,UiO-66@GO/PES膜的纯水通量分别增加了351% 和78%。Zr-MOFs复合膜水渗透性增加的原因包括两方面:首先,Zr-MOFs复合膜的亲水性更强,促进了水分子由膜面向膜内的传输[43]。其次,多孔Zr-MOFs改变了复合膜的结构,为水分子提供了额外的传输通道[58]。
4.3 抗污染性水处理过程中的膜污染包括有机污染、无机污染和微生物及其代谢产物导致的生物污染。研究者以牛血清白蛋白等大分子物质为模拟污染物,通过测定Zr-MOFs复合膜的通量恢复率(FRR)和阻力分布评估复合膜的抗污染性[59-60]。Dehghankar等[60]对PVDF膜和UiO-66/PVDF膜的抗污染性能进行了对比,结果显示,PVDF膜的FRR为47%,而UiO-66/PVDF膜的最佳FRR为71.8%。由此可见,Zr-MOFs复合膜具有良好的抗有机污染性能,这是因为Zr-MOFs复合膜通常具有较低的表面粗糙度和较高的亲水性,可以阻止污染物在膜表面的聚集和嵌入[43, 61]。目前,Zr-MOFs复合膜的抗污染性主要围绕有机污染展开,对水处理膜应用过程中常见的无机污染和生物污染还缺乏研究。
5 Zr-MOFs复合膜的水处理效能Zr-MOFs复合膜对水中重金属、油类和染料等污染物以及盐离子等物质的分离效能如表 2所示。
|
|
表 2 典型Zr-MOFs复合膜的水处理性能 Table 2 Performance of typical Zr-MOFs membranes in water treatment |
Zr-MOFs复合膜对水中常见重金属(Cr、As和Pb等)的去除率超过80%[9, 12, 33, 45, 65-68]。He等[33]测试了SGO@UiO-66-TFN膜对水中Cu(Ⅱ)和Pb(Ⅱ)的去除效能,结果显示,初始质量浓度为2 000 mg·L−1的Cu(Ⅱ)和Pb(Ⅱ)的去除率分别为99.4% 和97.5%。
Zr-MOFs复合膜对重金属离子的去除机制涉及吸附、道南效应和筛分等。当复合膜的孔径较大时[9, 66-67],吸附作用是重金属离子的主要的去除机制。Zhang等[45]认为带正电荷的UiO-66-NH2/PVDF膜对水溶液中Cr(Ⅵ)的高效去除是由于Cr(Ⅵ)与UiO-66-NH2之间的静电吸引。此外,Zr-MOFs纤维膜也可通过吸附作用去除重金属离子(见图 2)[18]。当复合膜的孔径较小时,重金属离子的去除主要依赖筛分和空间位阻效应[12, 33]。He等[33]发现,UiO-66的引入使PA层具有更多微小的孔隙(0.6 nm),可以有效拦截重金属离子。此外,他们认为在SGO@UiO-66-TFN膜表面形成的水化层可以有效阻止污染物与膜表面接触,强化重金属离子的去除效果。
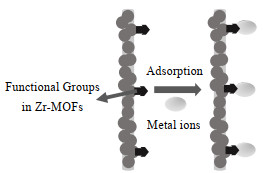
|
图 2 Zr-MOFs纤维膜对金属离子的吸附过程[18] Fig.2 Possible metal ion adsorption process of the Zr-MOFs fibrous membrane[18] |
虽然,Zr-MOFs具有较高的重金属去除效能,但是在重金属废水的处理中通常涉及多种重金属离子共存的情况,因此需要对重金属离子的混合物进行有效分离,目前仅有少数研究关注到了Zr-MOFs复合膜对混合重金属离子的去除效能,后续还需继续拓展。
5.2 油类污染物具有超亲水性和水下超疏油性的Zr-MOFs复合膜不仅对油类污染物的分离效果好(大于99%),还具有良好的抗污染性能[11, 24, 33, 42, 46, 48, 59]。但是,膜面缺陷会导致油滴进入支撑层,造成严重的膜污染。因此,无缺陷分离层的构建是目前Zr-MOFs油水分离膜的制备中遇到的重要挑战。Zhang等[46]利用PAA增强GO与UiO-66-NH2之间的相互作用,减少UiO-66-NH2/GO复合膜的表面缺陷,改性后的复合膜抗污染性能显著提高,通量下降率从56%降低到36%,FRR从67%增加到近100%。
Zr-MOFs复合膜在处理极端条件(高温、强酸碱性以及高盐)的含油废水中优势显著[42, 59]。通常Zr-MOFs无机复合膜更适合此条件下的油水分离。Zhu等[42]测试了铜网改性的UiO-66-NH2不锈钢膜对温度为−20~180 ℃的油水混合液的分离效能,结果显示,20个循环后,油的截留率仍保持在99% 以上。
5.3 染料含有UiO-66、UiO-66-NH2和UiO-66-(OH)2等Zr-MOFs的复合膜可以高效去除废水中的染料。用于去除染料的Zr-MOFs复合膜主要通过压力协助过滤或界面聚合法制备,此类复合膜具有致密表面,可以阻止染料分子跨膜运输,提升小分子染料的去除效能[19, 36, 57]。
复合膜对染料的去除机制涉及3种作用。首先,Zr-MOFs可以通过吸附作用截留染料[43]。然而吸附受限于位点的数量和聚合物基质。El Mehalmey等[14]发现UiO-66-NH2/CA复合膜中的聚合物基质阻止了阴离子染料甲基橙(MO)扩散到带正电的UiO-66-NH2笼形结构中(图 3(a))。其次,不同电性的染料和Zr-MOFs复合膜之间存在静电吸引或排斥作用[36, 50]。Aghili等[36]发现,由于MO具有较低的负电荷密度,UiO-66-NH2-TFN膜对MO的排斥力较低,使得MO的去除率低于刚果红和日落黄。此外,部分Zr-MOFs复合膜的孔径小于染料分子的尺寸,通过孔径筛分可以实现染料的高效去除[19, 69]。
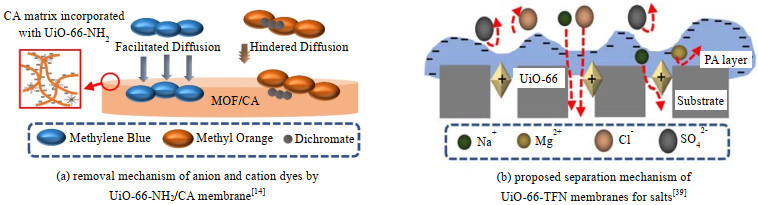
|
图 3 典型Zr-MOFs膜的结构示意图及污染物去除机制[14, 39] Fig.3 Schematic diagram of typical Zr-MOFs membranes structure and pollutant removal mechanism[14, 39] |
目前,Zr-MOFs复合膜对单一染料分子的去除率较高,但是,实际的染料废水水质复杂,在分离的过程中还需要考虑多种水质条件,如水中的共存物质和pH,而且涉及染料回收时还需要对不同的染料分子进行选择性分离,现有的研究中还缺乏相关的探讨与解析。
5.4 脱盐Zr-MOFs复合膜在脱盐中的应用主要通过反渗透(RO)、纳滤(NF)、正渗透(FO)、膜蒸馏和渗透汽化等工艺实现[13, 62-63, 70-71]。PCN-222、UiO-66等Zr-MOFs改性的TFN膜可以通过NF和RO实现高效脱盐,其中RO对所有盐离子均有较高的去除率[13, 25],而NF仅对二价盐离子有效[30-31]。TFN膜的纳滤截盐机制如图 3(b)所示,外层PA阻止高价离子通过,而内层Zr-MOFs可以进一步通过静电屏蔽和空间位阻实现盐离子的高效截留[39]。Liu等[51]发现,UiO-66-NH2-TFN膜的对于Na2SO4的最高截留率为99%,而对于NaCl的截留率仅为38.1%。与原始膜相比,含Zr-MOFs的TFN膜的水渗透性显著提高,而二价盐离子截留率基本保持不变[13, 70, 72]。但是,当Zr-MOFs分散性差或负载过量时,交联程度降低导致Zr-MOFs-TFN膜形成界面缺陷,使膜的渗透性增加,而盐截留率降低[29-30]。
Zr-MOFs-TFN膜也可用于FO脱盐[28, 31]。Zr-MOFs的加入改变了FO膜的纯水通量和选择性[31]。Liu等[28]在FO模式下测试了UiO-66复合膜的脱盐性能。结果显示,该膜的水/Na2SO4的选择性为13.5 g·L−1,水渗透性约为14.1 L·m−2·h−1·MPa−1,比聚合物膜高出约3倍和56倍。
5.5 其他污染物近年来,研究者也尝试将Zr-MOFs复合膜用于水中抗生素等新型污染物的去除[32, 72]。Guo等[32]将制备的UiO-66复合膜用于废水中四环素和四环素抗性基因的去除,结果显示,在FO操作模式下,该膜对四环素的截留率约为99%,而四环素抗性基因的渗透性低于10%。目前,仅有Zr-MOFs改性的FO膜用于此类新型污染物的去除中。研究显示,Zr-MOFs对抗生素等新型污染物有较强的吸附亲和力[73],所以其他种类的Zr-MOFs复合膜也可以尝试用于废水中药物类新型污染物的去除。
6 结论与展望Zr-MOFs复合膜具有良好的渗透性和抗污染性,不仅可以高效去除水中污染物,还可以通过选择分离实现有用组分的回收。“双碳”背景下,Zr-MOFs膜在水处理中显示出广阔的应用前景。然而,现有方法制得的Zr-MOFs复合膜存在负载量低、易产生表面缺陷和稳定性差等问题,目前主要用于实验室模拟废水的处理研究。为进一步拓展Zr-MOFs膜在水处理中的发展和实际应用,未来的研究可以考虑从以下方面进行:
(1) 开发Zr-MOFs复合膜的优化制备策略:深入解析Zr-MOFs和聚合物等成膜材料之间的相互作用机制及其对复合膜的表面粗糙度、亲疏水性等性质的影响规律,以此为基础,精准进行Zr-MOFs与成膜材料的配对,探索结构良好且稳定高效的Zr-MOFs复合膜的制备策略。
(2) 提升Zr-MOFs膜的选择分离性:Zr-MOFs的突出优点之一是选择分离性,但是目前仅在染料体系以及染料/盐体系进行研究,后续还可以进行重金属等体系的选择分离研究,此外,可以通过引入对特定污染物有亲和力的Zr-MOFs或调控复合膜表面的电荷分布实现目标污染物的高效选择和分离回收。
(3) 评价Zr-MOFs复合膜对实际废水处理的有效性:深入解析各种水质条件(如pH、盐度等)以及废水中其他组分对目标污染物去除的影响机制,并以实际废水作为进水水质,综合评估Zr-MOFs复合膜对该类废水处理的有效性。
| [1] |
李明俊. 2022年中国膜产业全景图谱[EB/OL]. (2022-06-08) [2023-05-03]. https://www.qianzhan.com/analyst/detail/220/220608-2a254a1b.html. LI M J. Panoramic map of China's membrane industry in 2022[EB/OL]. (2022-06-08) [2023-05-03]. https://www.qianzhan.com/analyst/detail/220/220608-2a254a1b.html. |
| [2] |
姚远, 杨彩虹, 吴礼光, 等. 聚偏氟乙烯/TiO2-石墨烯杂化膜的制备及其性能[J]. 高校化学工程学报, 2020, 34(1): 237-246. YAO Y, YANG C H, WU L G, et al. Preparation and performance of PVDF/TiO2-GO hybrid ultrafiltration membranes[J]. Journal of Chemical Engineering of Chinese Universities, 2020, 34(1): 237-246. DOI:10.3969/j.issn.1003-9015.2020.01.030 |
| [3] |
SCHNEEMANN A, BON V, SCHWEDLER I, et al. Flexible metal-organic frameworks[J]. Chemical Society Reviews, 2014, 43(16): 6062-6096. DOI:10.1039/C4CS00101J |
| [4] |
JAMES S L. Metal-organic frameworks[J]. Chemical Society Reviews, 2003, 32(5): 276-288. DOI:10.1039/b200393g |
| [5] |
HOU J, WANG H T, ZHANG H C, et al. Zirconium metal-organic framework materials for efficient ion adsorption and sieving[J]. Industrial & Engineering Chemistry Research, 2020, 59(29): 12907-12923. |
| [6] |
CHEN Z J, HANNA S L, REDFERN L R, et al. Reticular chemistry in the rational synthesis of functional zirconium cluster-based MOFs[J]. Coordination Chemistry Reviews, 2019, 386: 32-49. DOI:10.1016/j.ccr.2019.01.017 |
| [7] |
ZENG H J, YU Z X, SHAO L Y, et al. Ag2CO3@UiO-66-NH2 embedding graphene oxide sheets photocatalytic membrane for enhancing the removal performance of Cr(Ⅵ) and dyes based on filtration[J]. Desalination, 2020, 491: 114558. DOI:10.1016/j.desal.2020.114558 |
| [8] |
XU L Y, ZHENG Q, WANG Y Y, et al. A pillared double-wall metal-organic framework adsorption membrane for the efficient removal of iodine from solution[J]. Separation and Purification Technology, 2021, 274: 118436. DOI:10.1016/j.seppur.2021.118436 |
| [9] |
WAN P, YUAN M X, YU X L, et al. Arsenate removal by reactive mixed matrix PVDF hollow fiber membranes with UIO-66 metal organic frameworks[J]. Chemical Engineering Journal, 2020, 382: 122921. DOI:10.1016/j.cej.2019.122921 |
| [10] |
EFOME J E, RANA D, MATSUURA T, et al. Experiment and modeling for flux and permeate concentration of heavy metal ion in adsorptive membrane filtration using a metal-organic framework incorporated nanofibrous membrane[J]. Chemical Engineering Journal, 2018, 352: 737-744. DOI:10.1016/j.cej.2018.07.077 |
| [11] |
XUE J J, XU M J, GAO J M, et al. Multifunctional porphyrinic Zr-MOF composite membrane for high-performance oil-in-water separation and organic dye adsorption/photocatalysis[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 628: 127288. DOI:10.1016/j.colsurfa.2021.127288 |
| [12] |
HE M L, WANG L, LV Y T, et al. Novel polydopamine/metal organic framework thin film nanocomposite forward osmosis membrane for salt rejection and heavy metal removal[J]. Chemical Engineering Journal, 2020, 389: 124452. DOI:10.1016/j.cej.2020.124452 |
| [13] |
BONNETT B L, SMITH E D, DE LA GARZA M, et al. PCN-222 metal-organic framework nanoparticles with tunable pore size for nanocomposite reverse osmosis membranes[J]. ACS Applied Materials & Interfaces, 2020, 12(13): 15765-15773. |
| [14] |
EL-MEHALMEY W A, SAFWAT Y, BASSYOUNI M, et al. Strong interplay between polymer surface charge and MOF cage chemistry in mixed-matrix membrane for water treatment applications[J]. ACS Applied Materials & Interfaces, 2020, 12(24): 27625-27631. |
| [15] |
ZHANG H Z, SUN J Y, ZHANG Z L, et al. Hybridly charged NF membranes with MOF incorporated for removing low-concentration surfactants[J]. Separation and Purification Technology, 2021, 258: 118069. DOI:10.1016/j.seppur.2020.118069 |
| [16] |
CAVKA J H, JAKOBSEN S, OLSBYE U, et al. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability[J]. Journal of the American Chemical Society, 2008, 130(42): 13850-13851. DOI:10.1021/ja8057953 |
| [17] |
JASUJA H, PETERSON G W, DECOSTE J B, et al. Evaluation of MOFs for air purification and air quality control applications: Ammonia removal from air[J]. Chemical Engineering Science, 2015, 124: 118-124. DOI:10.1016/j.ces.2014.08.050 |
| [18] |
HUA W K, ZHANG T H, WANG M, et al. Hierarchically structural PAN/UiO-66-(COOH)2 nanofibrous membranes for effective recovery of Terbium(Ⅲ) and Europium(Ⅲ) ions and their photoluminescence performances[J]. Chemical Engineering Journal, 2019, 370: 729-741. DOI:10.1016/j.cej.2019.03.255 |
| [19] |
WANG X R, ZHAI L Z, WANG Y X, et al. Improving water-treatment performance of zirconium metal organic framework membranes by postsynthetic defect healing[J]. ACS Applied Materials & Interfaces, 2017, 9(43): 37848-37855. |
| [20] |
FENG D W, GU Z Y, LI J R, et al. Zirconium-metalloporphyrin PCN-222: Mesoporous metal-organic frameworks with ultrahigh stability as biomimetic catalysts[J]. Angewandte Chemie International Edition, 2012, 51(41): 10307-10310. DOI:10.1002/anie.201204475 |
| [21] |
MORRIS W, VOLOSSKIY B, DEMIR S, et al. Synthesis, structure, and metalation of two new highly porous zirconium metal-organic frameworks[J]. Inorganic Chemistry, 2012, 51(12): 6443-6445. DOI:10.1021/ic300825s |
| [22] |
FENG D W, CHUNG W C, WEI Z W, et al. Construction of ultrastable porphyrin Zr metal-organic frameworks through linker elimination[J]. Journal of the American Chemical Society, 2013, 135(45): 17105-17110. DOI:10.1021/ja408084j |
| [23] |
EFOME J E, RANA D, MATSUURA T, et al. Metal-organic frameworks supported on nanofibers to remove heavy metals[J]. Journal of Materials Chemistry A, 2018, 6(10): 4550-4555. DOI:10.1039/C7TA10428F |
| [24] |
CHEN X Y, CHEN D Y, LI N J, et al. Modified-MOF-808-loaded polyacrylonitrile membrane for highly efficient, simultaneous emulsion separation and heavy metal ion removal[J]. ACS Applied Materials & Interfaces, 2020, 12(35): 39227-39235. |
| [25] |
YIN B, SUN L W, TANG S K, et al. Preparation of metal-organic framework/polyvinylidene fluoride mixed matrix membranes for water treatment[J]. Industrial & Engineering Chemistry Research, 2020, 59(44): 19689-19697. |
| [26] |
SUN H Z, TANG B B, WU P Y. Development of hybrid ultrafiltration membranes with improved water separation properties using modified superhydrophilic metal-organic framework nanoparticles[J]. ACS Applied Materials & Interfaces, 2017, 9(25): 21473-21484. |
| [27] |
AL-SHAELI M, SMITH S J D, JIANG S X, et al. Long-term stable metal organic framework (MOF) based mixed matrix membranes for ultrafiltration[J]. Journal of Membrane Science, 2021, 635: 119339. DOI:10.1016/j.memsci.2021.119339 |
| [28] |
LIU T Y, YUAN H G, LIU Y Y, et al. Metal-organic framework nanocomposite thin films with interfacial bindings and self-standing robustness for high water flux and enhanced ion selectivity[J]. ACS Nano, 2018, 12(9): 9253-9265. DOI:10.1021/acsnano.8b03994 |
| [29] |
LIN Y Q, WU H C, SHEN Q, et al. Custom-tailoring metal-organic framework in thin-film nanocomposite nanofiltration membrane with enhanced internal polarity and amplified surface crosslinking for elevated separation property[J]. Desalination, 2020, 493: 114649. DOI:10.1016/j.desal.2020.114649 |
| [30] |
LIU H R, ZHANG M, ZHAO H, et al. Enhanced dispersibility of metal-organic frameworks (MOFs) in the organic phase via surface modification for TFN nanofiltration membrane preparation[J]. RSC Advances, 2020, 10(7): 4045-4057. DOI:10.1039/C9RA09672H |
| [31] |
BAGHERZADEH M, BAYRAMI A, AMINI M. Enhancing forward osmosis (FO) performance of polyethersulfone/polyamide (PES/PA) thin-film composite membrane via the incorporation of GQDs@UiO-66-NH2 particles[J]. Journal of Water Process Engineering, 2020, 33: 101107. DOI:10.1016/j.jwpe.2019.101107 |
| [32] |
GUO J L, HUANG M H, GAO P, et al. Simultaneous robust removal of tetracycline and tetracycline resistance genes by a novel UiO/TPU/PSF forward osmosis membrane[J]. Chemical Engineering Journal, 2020, 398: 125604. DOI:10.1016/j.cej.2020.125604 |
| [33] |
HE M L, WANG L, ZHANG Z, et al. Stable forward osmosis nanocomposite membrane doped with sulfonated graphene oxide@metal-organic frameworks for heavy metal removal[J]. ACS Applied Materials & Interfaces, 2020, 12(51): 57102-57116. |
| [34] |
BAGHERZADEH M, BAYRAMI A, AMINI M. Thin-film nanocomposite forward osmosis membranes modified with Zr-based metal-organic framework to improve desalination performance[J]. Applied Organometallic Chemistry, 2020, 34(2): 5339. DOI:10.1002/aoc.5339 |
| [35] |
MA D C, PEH S B, HAN G, et al. Thin-film nanocomposite (TFN) membranes incorporated with super-hydrophilic metal- organic framework (MOF) UiO-66: Toward enhancement of water flux and salt rejection[J]. ACS Applied Materials & Interfaces, 2017, 9(8): 7523-7534. |
| [36] |
AGHILI F, GHOREYSHI A A, VAN DER BRUGGEN B, et al. Introducing gel-based UiO-66-NH2 into polyamide matrix for preparation of new super hydrophilic membrane with superior performance in dyeing wastewater treatment[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105484. DOI:10.1016/j.jece.2021.105484 |
| [37] |
LIU L F, XIE X, QI S R, et al. Thin-film nanocomposite reverse osmosis membrane incorporated with UiO-66 nanoparticles for enhanced boron removal[J]. Journal of Membrane Science, 2019, 580: 101-109. DOI:10.1016/j.memsci.2019.02.072 |
| [38] |
HE Y R, TANG Y P, MA D C, et al. UiO-66 incorporated thin-film nanocomposite membranes for efficient selenium and arsenic removal[J]. Journal of Membrane Science, 2017, 541: 262-270. DOI:10.1016/j.memsci.2017.06.061 |
| [39] |
GU Z Y, YU S L, ZHU J Y, et al. Incorporation of lysine-modified UiO-66 for the construction of thin-film nanocomposite nanofiltration membrane with enhanced water flux and salt selectivity[J]. Desalination, 2020, 493: 114661. DOI:10.1016/j.desal.2020.114661 |
| [40] |
LIU X L, DEMIR N K, WU Z T, et al. Highly water-stable zirconium metal organic framework UiO-66 membranes supported on alumina hollow fibers for desalination[J]. Journal of the American Chemical Society, 2015, 137(22): 6999-7002. DOI:10.1021/jacs.5b02276 |
| [41] |
ABDULLAH N, RAHMAN M A, OTHMAN M H D, et al. Preparation, characterizations and performance evaluations of alumina hollow fiber membrane incorporated with UiO-66 particles for humic acid removal[J]. Journal of Membrane Science, 2018, 563: 162-174. DOI:10.1016/j.memsci.2018.05.059 |
| [42] |
ZHU M, LIU Y C, CHEN M Y, et al. Robust superhydrophilic and underwater superoleophobic membrane optimized by Cu doping modified metal-organic frameworks for oil-water separation and water purification[J]. Journal of Membrane Science, 2021, 640: 119755. DOI:10.1016/j.memsci.2021.119755 |
| [43] |
LIU Y C, GAN D, CHEN M Y, et al. Bioinspired dopamine modulating graphene oxide nanocomposite membrane interposed by super-hydrophilic UiO-66 with enhanced water permeability[J]. Separation and Purification Technology, 2020, 253: 117552. DOI:10.1016/j.seppur.2020.117552 |
| [44] |
TRINH D X, TANIKE T, TOSHIAKI T. Fabrication of new composite membrane filled with UiO-66 nanoparticles and its application to nanofiltration[J]. Separation and Purification Technology, 2017, 177: 249-256. DOI:10.1016/j.seppur.2017.01.004 |
| [45] |
ZHANG Y H, XU X M, YUE C L, et al. Insight into the efficient co-removal of Cr(Ⅵ) and Cr(Ⅲ) by positively charged UiO-66-NH2 decorated ultrafiltration membrane[J]. Chemical Engineering Journal, 2021, 404: 126546. DOI:10.1016/j.cej.2020.126546 |
| [46] |
ZHANG R N, CAO J L, LIU Y N, et al. Metal-organic framework-intercalated graphene oxide membranes for highly efficient oil/water separation[J]. Industrial & Engineering Chemistry Research, 2020, 59(38): 16762-16771. |
| [47] |
ZENG H J, YU Z X, SHAO L Y, et al. A novel strategy for enhancing the performance of membranes for dyes separation: Embedding PAA@UiO-66-NH2 between graphene oxide sheets[J]. Chemical Engineering Journal, 2021, 403: 126281. DOI:10.1016/j.cej.2020.126281 |
| [48] |
CAO J L, SU Y L, LI Y N, et al. Self-assembled MOF membranes with underwater superoleophobicity for oil/water separation[J]. Journal of Membrane Science, 2018, 566: 268-277. DOI:10.1016/j.memsci.2018.08.068 |
| [49] |
SHI P, HU X K, DUAN M. A UiO-66/tannic acid/chitosan/polyethersulfone hybrid membrane-like adsorbent for the dynamic removal of dye and Cr(Ⅵ) from water[J]. Journal of Cleaner Production, 2021, 290: 125794. DOI:10.1016/j.jclepro.2021.125794 |
| [50] |
ZHANG P, GONG J L, ZENG G M, et al. Ultrathin reduced graphene oxide/MOF nanofiltration membrane with improved purification performance at low pressure[J]. Chemosphere, 2018, 204: 378-389. DOI:10.1016/j.chemosphere.2018.04.064 |
| [51] |
LIU H R, GAO J, LIU G H, et al. Enhancing permeability of thin film nanocomposite membranes via covalent linking of polyamide with the incorporated metal-organic frameworks[J]. Industrial & Engineering Chemistry Research, 2019, 58(20): 8772-8783. |
| [52] |
MATEBESE F, MOUTLOALI R M. Greywater reclamation: A comparison of the treatment performance of UiO-66-NH2@GO nanocomposites membrane filtration with and without activated carbon pretreatment[J]. Journal of Environmental Chemical Engineering, 2021, 9(1): 104906. DOI:10.1016/j.jece.2020.104906 |
| [53] |
YANG S J, ZOU Q F, WANG T H, et al. Effects of GO and MOF@GO on the permeation and antifouling properties of cellulose acetate ultrafiltration membrane[J]. Journal of Membrane Science, 2019, 569: 48-59. DOI:10.1016/j.memsci.2018.09.068 |
| [54] |
LIU Y C, TU W W, CHEN M Y, et al. A mussel-induced method to fabricate reduced graphene oxide/halloysite nanotubes membranes for multifunctional applications in water purification and oil/water separation[J]. Chemical Engineering Journal, 2018, 336: 263-277. DOI:10.1016/j.cej.2017.12.043 |
| [55] |
PEETERS J M M, BOOM J P, MULDER M H V, et al. Retention measurements of nanofiltration membranes with electrolyte solutions[J]. Journal of Membrane Science, 1998, 145(2): 199-209. DOI:10.1016/S0376-7388(98)00079-9 |
| [56] |
谢艳新, 唐玉岭, 杨倩, 等. 不同荷电性聚氯乙烯超滤膜制备及性能[J]. 高校化学工程学报, 2020, 34(6): 1527-1535. XIE Y X, TANG Y L, YANG Q, et al. Preparation and performance of different charged polyvinyl chloride ultrafiltration membranes[J]. Journal of Chemical Engineering of Chinese Universities, 2020, 34(6): 1527-1535. |
| [57] |
MA J, GUO X Y, YING Y P, et al. Composite ultrafiltration membrane tailored by MOF@GO with highly improved water purification performance[J]. Chemical Engineering Journal, 2017, 313: 890-898. DOI:10.1016/j.cej.2016.10.127 |
| [58] |
ZHAO P X, LI R, WU W J, et al. In-situ growth of polyvinylpyrrolidone modified Zr-MOFs thin-film nanocomposite (TFN) for efficient dyes removal[J]. Composites Part B: Engineering, 2019, 176: 107208. DOI:10.1016/j.compositesb.2019.107208 |
| [59] |
SAMARI M, ZINADINI S, ZINATIZADEH A A, et al. Designing of a novel polyethersulfone (PES) ultrafiltration (UF) membrane with thermal stability and high fouling resistance using melamine-modified zirconium-based metal-organic framework (UiO-66-NH2/MOF)[J]. Separation and Purification Technology, 2020, 251: 117010. DOI:10.1016/j.seppur.2020.117010 |
| [60] |
DEHGHANKAR M, MOHAMMADI T, MOGHADAM M T, et al. Metal-organic framework/zeolite nanocrystal/polyvinylidene fluoride composite ultrafiltration membranes with flux/antifouling advantages[J]. Materials Chemistry and Physics, 2021, 260: 124128. DOI:10.1016/j.matchemphys.2020.124128 |
| [61] |
LIANG Z H, WANG J, ZHANG Q Y, et al. Composite PVDF ultrafiltration membrane tailored by sandwich-like GO@UiO-66 nanoparticles for breaking the trade-off between permeability and selectivity[J]. Separation and Purification Technology, 2021, 276: 119308. DOI:10.1016/j.seppur.2021.119308 |
| [62] |
LI H T, FU M, WANG S Q, et al. Stable Zr-based metal–organic framework nanoporous membrane for efficient desalination of hypersaline water[J]. Environmental Science & Technology, 2021, 55(21): 14917-14927. |
| [63] |
CHEN L, LI F Q, JIANG L J, et al. UiO-66-NH2/PVA composite Janus membrane with a dense hydrophilic surface layer for strong resistance to fouling and wettability in membrane distillation[J]. Journal of Water Process Engineering, 2022, 48: 102887. DOI:10.1016/j.jwpe.2022.102887 |
| [64] |
MU T W, ZHANG Y Y, SHI W, et al. A novel UiO-66/PSF-composite membrane for the rejection of multiple antibiotics: Numerical simulation and experiment verification[J]. Chemosphere, 2021, 269: 128686. DOI:10.1016/j.chemosphere.2020.128686 |
| [65] |
EFOME J E, RANA D, MATSUURA T, et al. Effects of operating parameters and coexisting ions on the efficiency of heavy metal ions removal by nano-fibrous metal-organic framework membrane filtration process[J]. Science of the Total Environment, 2019, 674: 355-362. DOI:10.1016/j.scitotenv.2019.04.187 |
| [66] |
JAMSHIDIFARD S, KOUSHKBAGHI S, HOSSEINI S, et al. Incorporation of UiO-66-NH2 MOF into the PAN/chitosan nanofibers for adsorption and membrane filtration of Pb(Ⅱ), Cd(Ⅱ) and Cr(Ⅵ) ions from aqueous solutions[J]. Journal of Hazardous Materials, 2019, 368: 10-20. DOI:10.1016/j.jhazmat.2019.01.024 |
| [67] |
EFOME J E, RANA D, MATSUURA T, et al. Insight studies on metal-organic framework nanofibrous membrane adsorption and activation for heavy metal ions removal from aqueous solution[J]. ACS Applied Materials & Interfaces, 2018, 10(22): 18619-18629. |
| [68] |
PISHNAMAZI M, KOUSHKBAGHI S, HOSSEINI S S, et al. Metal organic framework nanoparticles loaded-PVDF/chitosan nanofibrous ultrafiltration membranes for the removal of BSA protein and Cr(Ⅵ) ions[J]. Journal of Molecular Liquids, 2020, 317: 113934. DOI:10.1016/j.molliq.2020.113934 |
| [69] |
AGHILI F, GHOREYSHI A A, RAHIMPOUR A, et al. New chemistry for mixed matrix membranes: Growth of continuous multilayer UiO-66-NH2 on UiO-66-NH2-based polyacrylonitrile for highly efficient separations[J]. Industrial & Engineering Chemistry Research, 2020, 59(16): 7825-7838. |
| [70] |
ZHU J Y, HOU J W, YUAN S S, et al. MOF-positioned polyamide membranes with a fishnet-like structure for elevated nanofiltration performance[J]. Journal of Materials Chemistry A, 2019, 7(27): 16313-16322. DOI:10.1039/C9TA02299F |
| [71] |
WAN L L, ZHOU C, XU K, et al. Synthesis of highly stable UiO-66-NH2 membranes with high ions rejection for seawater desalination[J]. Microporous and Mesoporous Materials, 2017, 252: 207-213. DOI:10.1016/j.micromeso.2017.06.025 |
| [72] |
WANG Z Y, QI J Y, LU X H, et al. Epitaxially grown MOF membranes with photocatalytic bactericidal activity for biofouling mitigation in desalination[J]. Journal of Membrane Science, 2021, 630: 119327. DOI:10.1016/j.memsci.2021.119327 |
| [73] |
赵英杰, 赵慧芳, 王婷, 等. 金属-有机骨架材料用于水中痕量药物污染物的吸附脱除[J]. 化工进展, 2020, 39(6): 2187-2205. ZHAO Y J, ZHAO H F, WANG T, et al. Adsorption removal of trace pharmaceutical pollutants from water by metal-organic frameworks[J]. Chemical Industry and Engineering Progress, 2020, 39(6): 2187-2205. |