2. 浙江大学 化学工程与生物工程学院,浙江 杭州 310027
2. College of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310027, China
化石燃料燃烧排放的氮氧化物(NOx,主要包括NO、NO2、N2O等)是主要的大气污染物之一。NOx进入大气环境后,易造成细颗粒物污染、光化学烟雾、酸雨等环境问题。随着我国机动车保有量增加,机动车对柴油和汽油的消耗量持续攀升,致使机动车NOx排放量逐年增大。因此,机动车已成为我国NOx的主要排放源之一。据《2018年中国机动车环境管理年报》统计,2017年我国机动车NOx排放量为574.3万吨,其中柴油车排放的NOx占我国机动车NOx排放总量的70%左右。因此,柴油车已成为机动车NOx排放的主要贡献源。2014年出台的《城市车辆用柴油发动机排气污染物排放限值及测量方法(HJ 689-2014)》对柴油车排放的NOx浓度限值提出更加严格的标准,第四阶段NOx排放限值为4.20 g·kW-1h-1,第五阶段要降至2.80 g·kW-1h-1。因此,为改善我国城市大气环境质量,急需采取有效措施控制柴油车的NOx排放。
柴油车排放的NOx主要是柴油燃烧过程中N2与O2在高温条件下反应生成的,约90%的NOx为NO。柴油车采用稀薄燃烧技术提高燃油效率的同时,也使尾气含氧量增高,传统的“三效催化剂”不能有效处理富氧环境中的NOx。目前,柴油发动机NOx排放控制技术主要包括:储存还原(LNTs)技术,碳氢化合物选择性催化还原(HC-SCR)技术和氨选择性催化还原(NH3-SCR)技术。其中NH3-SCR技术被认为是最有效的NOx减排技术。针对柴油车尾气,NH3-SCR技术由尿素溶液热解提供氨源[1-2],利用NH3为还原剂,优先将富氧环境中的NOx催化还原为N2和H2O[3],如图 1所示。NH3选择性催化还原NOx的标准反应如式(1)所示[4]。
| $ \text{4N}{{\text{H}}_{\text{3}}}\text{+4NO+}{{\text{O}}_{\text{2}}}\to \text{4}{{\text{N}}_{\text{2}}}\text{+6}{{\text{H}}_{\text{2}}}\text{O} $ | (1) |
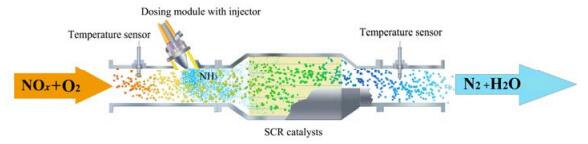
|
图 1 柴油机NH3-SCR净化器示意图 Fig.1 Schematic diagram of NH3-SCR reactor of diesel engine |
SCR催化剂是NH3-SCR技术的核心,随着柴油车国V排放标准的实施,柴油车SCR催化剂市场将迎来爆发式增长,研发高效SCR催化剂具有重要意义和价值。本文围绕以氨为还原剂的NH3-SCR技术,分析不同类型催化剂催化活性的差异,阐述催化剂表面NH3选择性催化还原NOx反应机理,探讨SCR催化剂抗中毒性能,总结并展望柴油车SCR催化剂的研究方向。
2 NH3-SCR催化剂研究进展NH3-SCR催化剂主要包括金属氧化物催化剂和分子筛催化剂。金属氧化物催化剂由V、Mn、Ce、Cu、Fe等活性组分负载在Al2O3、TiO2等载体上制备而得;分子筛催化剂由Ce、Cu、Fe等活性组分负载在ZSM-5分子筛、BEA分子筛(也称为beta分子筛)、SAPO-34分子筛等载体上制备而得。围绕上述两类催化剂的催化特性,学者们就其催化性能做了大量研究。
2.1 金属氧化物催化剂钒(V)氧化物催化剂是较早被运用于净化发动机尾气的NH3-SCR催化剂,钨(W)和钼(Mo)通常被添加到钒氧化物催化剂中,以提高其催化活性。其中V2O5-WO3/TiO2是商业化开发最为成熟的钒基NH3-SCR催化剂。钒氧化物催化剂虽然生产成本低、抗硫性好,但也具有很多不足,如低温催化活性不高,易将SO2催化氧化为SO3,热稳定性差,具有生物毒性,废弃催化剂处置困难等[5]。因此,有必要开发廉价、无毒、高效的氧化物催化剂来替代钒氧化物催化剂。铁(Fe)氧化物、锰(Mn)氧化物和铈(Ce)氧化物催化剂表现出了良好的催化活性,是近年来NH3-SCR催化剂的热点研究对象。
铁在地壳中储量丰富,Fe3+和Fe2+间的转换有利于氧化还原反应的进行。FeOx掺杂Nb2O5(五氧化二铌)能增加催化剂表面的酸性位点,并提高催化剂的NH3-SCR催化活性[6]。通过共沉淀法制备的FeW催化剂在250~450 ℃的NOx转化率能够维持在100%,分析表明,掺杂W能够增加催化剂表面的酸性位,其中Brönsted酸性位主要由W-OH结构供给,而Lewis酸性位主要由铁氧化物提供[7]。虽然铁氧化物催化剂具备较好的中高温催化活性,但其低温NH3-SCR催化活性还有待提高。
锰氧化物种类多,反应过程中Mn价态间的变化,有利于NH3-SCR反应,因此锰氧化物催化剂具备极好的低温催化活性。SUN等[8]利用共沉淀法制备了MnEuOx-0.1催化剂,体积空速(GHSV)为108 000 h-1时,在150~400 ℃的NOx转化率能保持在100%左右。通过共沉淀法合成的Fe0.3Mn0.5Zr0.2催化剂在200~360 ℃的NOx转化率能维持在100%左右[9]。FANG等[10]的研究表明,Mg能够嵌入Mnx/TiO2催化剂的MnOx晶格中形成MgMn2O4,而MgMn2O4能够提高催化剂在NH3-SCR反应中的催化活性和稳定性。Mn/Ce/TiW催化剂180 ℃的NOx去除率为90%,其催化活性与表面酸性、还原能力、活性组分含量和分散度有关[11]。具有纳米微球形貌的MnOx-CeO2催化剂具备良好的低温催化活性和抗硫性[12]。可见,掺杂元素、载体、活性组分形貌和合成方法均会对锰氧化物催化剂的催化活性产生影响。柴油车尾气温度因柴油发动机运行的工况不同而存在较大的波动,如何在维持锰氧化物催化剂良好的低温催化活性的同时,提高其高温区间的NH3-SCR催化活性还需进一步研究。
CeO2 (氧化铈)是一种无毒的稀土氧化物,具备优异的储释氧性能,被广泛应用于制备NH3-SCR催化剂。研究表明,掺杂钼、钨、铌等过渡金属,能增强CeO2的表面酸性,提高其NH3-SCR催化活性[13-14]。CeO2表面Ce4+和Ce3+之间的可逆转化产生的氧空位,不仅能促进NH3-SCR催化反应,而且能催化氧化CO和HCs(碳氢化合物)[15]。掺杂Cu能使CeO2表面形成更多的氧空位,提高催化剂在NH3-SCR中的催化活性[16-17]。CeO2表面引入SO42-、硅钨酸、磷钼酸后,Lewis酸性位和Brönsted酸性位的数量和强度均得到增强,增加了催化剂对NH3的吸附能力,从而提高铈基氧化物催化剂在NH3-SCR反应中的催化活性[18-19]。可见,调控CeO2表面酸性位是改善其催化活性的重要手段。目前针对改性CeO2的研究,多侧重于活性评价和表面酸性变化,但改性对酸性位形成机制的影响规律尚不明确,导致选择改性试剂时存在一定的盲目性。因此,有必要进一步探明掺杂组分与CeO2之间的作用方式对酸性位形成机制的影响规律,从而有效提高铈基催化剂的SCR性能。
2.2 分子筛催化剂20世纪80年代,研究表明Cu-ZSM-5催化剂具备高效的NO分解和NOx选择性催化还原活性[20-21]。铜离子交换BEA分子筛催化剂的NH3-SCR催化活性和水热稳定性均优于Cu-ZSM-5分子筛[22]。Cu-BEA分子筛催化剂在200~350 ℃的NOx转化率和N2选择性分别维持在80%和95%以上[23]。近年来,铜离子交换菱沸石(CHA)结构的分子筛(如SAPO-34、SSZ-13)不仅催化活性温度窗口宽,而且呈现出比ZSM-5和BEA分子筛更优异的水热稳定性[24]。相对ZSM-5和BEA分子筛而言,CHA型分子筛的孔径较小,约为3.8 Å,小孔径对其SCR催化活性和水热稳定性起着关键作用[25]。KWAK等[24]通过对比Cu-SSZ-13、Cu-ZSM-5和Cu-beta三种类型催化剂的催化活性后,指出Cu-SSZ-13的催化活性温度窗口、NOx转化率和N2选择性均优于Cu-ZSM-5和Cu-beta催化剂。ZHAO等[26]合成的Cu-exchanged Al-rich SSZ-13催化剂在150~650 ℃的催化活性能维持85%以上(GHSV=80 000 h-1)。除铜基分子筛催化剂外,铁基分子筛催化剂和铈基分子筛催化剂也具备良好的NH3-SCR催化活性。IWASAKI等[27]利用离子交换法、浸渍法和气相沉积法制备了三种Fe-ZSM-5催化剂,表征分析结果表明Fe主要以FexOy低聚体、α-Fe2O3颗粒和Fe3+离子三种形态存在,其中离子交换形成的Fe3+是主要的活性中心。FREY等[28]的研究表明,相对于Fe-ZSM-5,Fe-BEA具备更高的NH3-SCR催化活性。活性金属负载量会对HBEA分子筛(酸型BEA分子筛)催化剂的催化活性产生影响,CeO2负载量越高,Ce/HBEA催化剂的催化氧化能力越强,高温条件下易造成NH3的过度氧化,降低NOx转化率[29]。
除负载单金属的分子筛催化剂外,利用活性金属间的协同催化作用,双金属或多金属负载的分子筛催化剂也是研究热点。ZHAO等[30]研究了添加Ce和Nb对Cu-beta分子筛催化剂催化活性的影响,结果表明添加Ce和Nb能够提高Cu-beta分子筛催化剂的催化活性。LIU等[31]制备了Mn-Ce/Cu-SSZ-13催化剂,利用Mn-Ce-Cu的协同作用,提高了该催化剂的低温催化活性,125~450 ℃时Mn-Ce/Cu-SSZ-13的NOx转化率能够维持在90%以上。CHI等[32]合成了以CeO2为核、Fe-ZSM-5为壳的核壳结构催化剂CeO2@Fe-ZSM-5,该催化剂在300~500 ℃的℃催化活性极好,且具有良好的抗水、抗硫性能。为提高Fe-ZSM-5的低温NH3-SCR催化活性,ZHANG等[33]制备了以Fe-ZSM-5为核、Ce负载介孔硅为壳的核壳结构催化剂Fe-ZSM-5@Ce/介孔硅,Ce/介孔硅壳层能够促进NO催化氧化为NO2,从而促进低温条件下的快速SCR反应。通过在Cu-SAPO-18外表面覆盖一层CeO2膜,能够同时提高催化剂的水热稳定性和抗硫性[34]。可见,通过多种金属的协同作用,能够显著改善催化剂的催化活性;控制催化剂的微观形貌也能调控其催化活性。然而,催化反应过程中活性金属间的相互作用机制,以及催化剂的微观形貌与其活性的关联效应还需进一步阐明。
2.3 其他催化剂天然矿石不仅含有多种金属元素,而且价格低廉。近年来,橄榄石、沸石、钙钛矿等矿石也被用于制备NH3-SCR催化剂[35-37]。遵循“以废治废”的思路,研究者也致力于利用废弃生物质制备活性炭NH3-SCR催化剂,实现废弃生物质的资源化利用[38-39]。利用马尾藻制备活性炭,并用作NH3-SCR催化剂,提高了马尾藻的附加值[40]。虽然利用生物质活性炭作为NH3-SCR催化剂能够实现资源循环利用,但是该类催化剂的催化活性较差、活性温度窗口较窄、催化剂易因氧化而损耗。柴油车尾气温度和含氧量均较高,生物质活性炭催化剂的热稳定性差,较难在此种条件下得到运用。
3 催化反应机理NOx的NH3-SCR反应属于多相催化反应,涉及到催化剂表面反应物和产物之间的化学键断裂和形成过程。研究表明,NH3-SCR催化反应机理主要分为Eley-Rideal (E-R)反应机理和Langmuir-Hinshelwood (L-H)反应机理。E-R反应机理是指气相中的NO与催化剂表面酸性位上吸附的NH3反应;L-H反应机理是指吸附的NO与催化剂表面酸性位上吸附的NH3反应。无论是E-R反应机理还是L-H反应机理,O2均参与催化反应,实现催化剂储、释氧催化循环过程[41-44]。可见,影响NH3-SCR催化效率的关键因素主要有以下两个:一个是催化剂表面储、释氧能力;另一个是催化剂表面吸附NH3的酸性位。催化剂表面储、释氧能力与活性金属的价态变化相关,如传统的钒基催化剂通过V5+与V4+间的氧化还原循环可实现催化剂的储、释氧过程[45-46];催化剂表面的酸性位主要分为Brönsted酸性位和Lewis酸性位,Brönsted酸性位可提供质子,与NH3结合形成吸附态的NH4+[47],Lewis酸性位可接受电子,与NH3结合形成-NH2基团[48],如图 2所示。
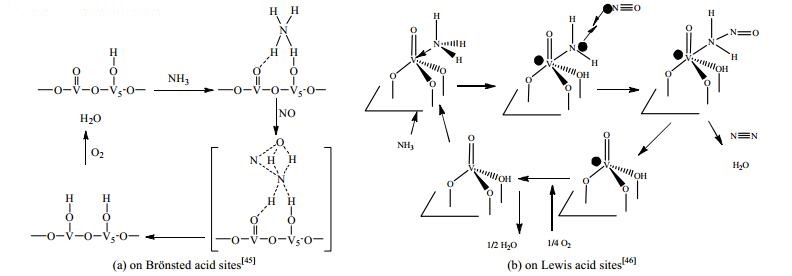
|
图 2 氧化钒表面Brönsted酸性位和Lewis酸性位上的NH3选择性催化还原NO反应的路径图 Fig.2 Mechanism of NH3-SCR of NO on Brönsted and Lewis acid sites over vanadium oxide surface |
SUN等[8]研究表明MnEuOx催化剂中,Eu(铕)不仅能够抑制MnOx晶化,而且能提高催化剂的还原能力和增加表面化学吸附态氧的数量;当反应温度低于150 ℃ ℃时,MnEuOx催化剂表面主要是以L-H反应机理为主,当反应温度高于150 ℃ ℃时,MnEuOx催化剂表面主要是以E-R反应机理为主。NAM等[49]利用原位红外表征(DRIFT)技术研究了W对Mn/Ce/Ti催化剂低温活性的影响,结果表明,添加W能够增加催化剂表面的Brönsted酸性位,形成更多的活性吸附态氨,如NH4+,并减少非活性的氮氧化物基团吸附。Ce0.75Zr0.25O2催化剂表面既存在E-R反应机理,也存在L-H反应机理[50],而CeO2-WO3表面主要是E-R反应机理[51]。BOROŃ等[52]研究了CoAlBEA催化剂表面的催化反应机理,指出BEA分子筛骨架外的Co基团比骨架中Co(II)离子的催化活性好,BEA分子筛骨架外Co基团上的SCR反应机理为E-R反应机理。CORTÉS-REYES等[53]利用水热法合成了Cu-SAPO-34催化剂,分子筛孔道内部的Cu2+在500 ℃℃时易被还原为Cu+,Cu2+/Cu+间的氧化还原循环途径对催化剂的SCR催化活性起着至关重要的作用。LIU等[31]指出Cu-SSZ-13催化剂上负载Mn-Ce后,在催化剂表面的NOx吸附形态由桥式双齿配位态变为更具低温活性的螯合单齿配位态,从而提高Cu-SSZ-13催化剂的低温催化活性;110 ℃时催化剂Mn-Ce/Cu-SSZ-13表面的催化反应机理为E-R反应机理。
综上所述,催化反应机理与催化剂的组成、催化反应温度相关。研究催化反应机理要结合催化剂本身的特点和实验条件进行相关分析。虽然研究者借助DRIFT技术鉴别催化剂表面NH3-SCR催化反应中间产物基团,以提高催化反应途径推导的可靠性,但是催化反应过程中电子的迁移转化途径还未研究清楚。今后,可利用表征结果,结合密度泛函理论(DFT)研究深层次的催化反应机理,以便设计出更加高效的NH3-SCR催化剂。
4 催化剂中毒机制 4.1 积炭中毒柴油车尾气中的主要污染物除NOx外,还含有颗粒物(PM)。我国柴油车排放的PM超过汽车排放总量的90%。SCR技术无法去除PM,而且PM的存在也会影响SCR催化剂的催化活性,需要耦合其他后处理技术来控制柴油车污染物PM的排放,防止PM在SCR催化剂表面沉积,导致SCR催化剂积炭失活。柴油车颗粒物过滤器(Diesel Particulate Filter, DPF)能有效处理PM,DPF一般放置在SCR装置前段,先通过物理作用过滤柴油车尾气中的PM,然后通过化学反应将DPF捕集到的PM氧化为CO2和H2O等气体。
除PM外,柴油车尾气中还含有未完全燃烧的C3H6和C3H8等碳氢化合物(HCs),HCs与SCR催化剂接触后易在催化剂表面形成积炭,覆盖NH3-SCR催化反应活性位点,堵塞孔道,造成催化剂积炭失活[54-56]。研究积炭对NH3-SCR催化剂催化活性的影响,有助于开发高效、耐久的NH3-SCR催化剂。ZSM-5和BEA分子筛表面酸性位点多,易吸附HCs并形成积炭。因此,ZSM-5和BEA分子筛催化剂的NH3-SCR催化活性易受HCs影响[30, 57]。通过在HBEA分子筛催化剂表面负载CeO2,能够促进C3H6催化氧化为COx,显著改善HBEA分子筛催化剂的抗积炭性能,具有核壳结构的CeO2@HBEA催化剂有优异的抗积炭性能,如图 3所示[29]。相对于ZSM-5和HBEA分子筛,虽然小孔径的SSZ-13和SAPO-34催化剂显示出较好的抗积炭性能,但其NH3-SCR催化活性仍然会受到HCs的抑制。掺杂Ce能减少Cu-SAPO-34催化剂表面酸性位数量、降低Cu-SAPO-34催化剂的C3H6吸附容量,从而改善Cu-SAPO-34的抗积炭能力[58]。MA等[55]的研究指出,C3H6抑制Cu-SSZ-13催化剂NH3-SCR催化活性的方式有两种,一种是低温时C3H6与NOx在催化剂表面的竞争吸附,另一种是高温时C3H6在催化剂表面因不完全催化氧化形成积炭。为改善SSZ-13分子筛的抗积炭性能,ZHANG等[59]利用介孔硅酸盐包裹在介孔SSZ-13分子筛外表面,形成核壳结构的meso-SSZ-13@MAS,由于C3H6不会在介孔硅酸盐表面形成积炭,所以meso-SSZ-13@MAS具有较好的抗积炭能力。因此,可通过下述两种途径改善催化剂的抗积炭性能:一是通过在催化剂中掺杂活性金属组分,降低HCs在催化剂表面的吸附容量,促进HCs催化氧化为COx;二是通过在催化剂表面包裹一层抗积炭性能好的材料,研发具备核壳结构的NH3-SCR催化剂。
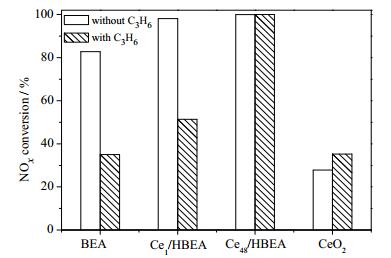
|
图 3 负载CeO2对HBEA分子筛NH3-SCR催化剂抗积炭性能的促进作用[29] Fig.3 Effects of CeO2 on propene poisoning resistance of HBEA zeolite NH3-SCR catalysts [29] |
柴油车尾气中的SO2会使SCR催化剂失活。按《车用柴油(V)GB 19147-2013》规定,我国现阶段车用柴油含硫量为50 mg·kg-1以下,2018年1月1日起,要求车用柴油含硫量低于10 mg·kg-1。SO2对NH3-SCR催化剂催化活性的影响一直是研究者关注的问题。SO2对NH3-SCR催化剂催化活性的影响主要包括如下两方面:一是低温时SO2与NH3反应生成的硫酸铵盐在催化剂表面沉积后会阻碍反应气体与催化剂表面活性中心接触,导致催化剂失活;二是SO2与SCR催化剂中的活性金属反应生成稳定的硫酸盐导致催化剂失活[58, 60-65]。SO2与Cu-SAPO-34催化剂表面的Cu2+反应生成铜的硫酸盐,降低铜离子的氧化还原性能、减少催化剂表面的活性位点数,同时反应气体中的NH3与SO2反应生成硫酸铵盐,也会降低催化剂活性,如图 4所示[66-67]。JIN等[64]的研究表明,SO2不仅会与Lewis酸性位上的Mn结合形成锰的硫酸盐,还会在Brönsted酸性位上形成NH4HSO4,降低Mn/TiO2催化剂的催化活性;Mn/TiO2掺杂Ce后,SO2易与Mn-Ce/TiO2催化剂表面的Ce反应生成铈的硫酸盐,从而减弱SO2对Mn活性基团的毒害作用,提高催化剂的抗硫性。FRANCE等[69]发现,Ce改性的FeMnOx催化剂具备良好的抗硫性,掺杂Ce能阻碍低温条件下NH4HSO4的生成。可见,通过在催化剂中掺杂Ce能够有效提高催化剂的抗硫性。
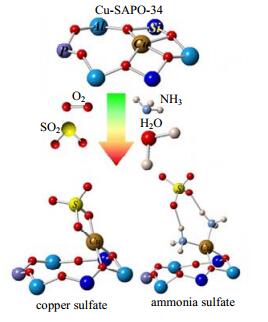
|
图 4 硫酸盐对Cu/SAPO-34催化剂NH3-SCR催化活性的影响[66] Fig.4 Effects of sulfate species on NH3-SCR activity of Cu/SAPO-34 [66] |
硫中毒催化剂的再生方法与催化剂的类型有关。硫中毒铈基催化剂可通过水洗法去除催化剂表面的硫酸盐,从而使其催化活性得到恢复[18];还可以通过高温(≥ 500 ℃)处理再生,该过程可与柴油车颗粒物过滤器催化剂的再生一起进行[68]。硫中毒铜基分子筛催化剂再生则可通过加入NH3、C3H6等还原剂在温度约400 ℃下进行[70]。
4.3 水热失活柴油车颗粒物过滤器再生时需将柴油车尾气温度升高到600 ℃以上[71],且柴油车尾气中含有水分,有必要考察催化剂的水热稳定性。研究表明当水热处理温度高于600 ℃600 ℃时,催化剂中的锐钛矿会转变为金红石,致使催化剂活性降低[72];当水热处理温度高于670 ℃670 ℃时,V4O10等会挥发,这类有毒物质进入大气环境后,会危害人体健康[73]。含Cu、Fe的ZSM-5和BEA分子筛催化剂虽然SCR催化活性高,但水热稳定性较差[74-78]。Cu-ZSM-5分子筛经700 ℃℃高温水热处理后,分子筛四面体骨架结构中的铝会变得不稳定并从结构中脱出,造成分子筛结构损坏,影响催化剂表面Brönsted酸性位和Cu离子位,从而降低催化剂的SCR催化活性[79]。通常认为,Al以Al(OH)3等形式从分子筛骨架中脱出是造成大孔分子筛结构损坏及催化活性下降的主要原因[76]。相对而言,SSZ-13和SAPO-34等CHA型分子筛的水热稳定性较好。由于Al(OH)3的直径比CHA分子筛的孔道尺寸大,无法从CHA分子筛结构中脱出,从而使CHA分子筛具有较好的水热稳定性,如图 5所示[80-82]。然而,当水热处理温度高于700 ℃℃时,SAPO-34和SSZ-13催化剂的催化活性同样会受到影响。LIU等[31]将Cu/SAPO-34催化剂在水蒸汽体积分数为10%的气氛中750 ℃水热处理24 h后,发现Cu/SAPO-34的催化活性温度窗口会移向高温区间[83]。Cu/SAPO-34催化剂的水热稳定性与其Cu含量有关,当Cu的质量分数高于1.7%时,催化剂易因结构坍塌而水热失活[84]。LEISTNER等[85]研究了550~850 ℃水热处理对Cu/SSZ-13催化剂的影响,当水热处理温度低于700 ℃700 ℃时,Cu/SSZ-13的SCR催化活性、NH3吸附容量和NH3氧化能力均不受明显影响;经850 ℃850 ℃水热处理后,SSZ-13分子筛骨架崩塌,导致其NH3-SCR催化活性显著降低。水热处理条件下,Cu-SSZ-13催化剂中分散的Cu2+离子会聚集生成CuO,破坏SSZ-13分子筛的骨架结构,Cu-SSZ-13催化剂的Si/Al比值越高,其水热稳定性越差。
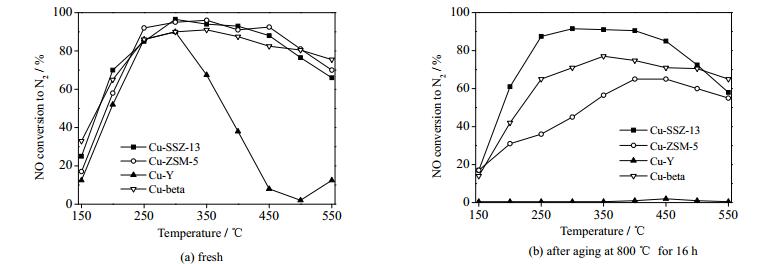
|
图 5 水热处理对不同类型铜基分子筛NH3-SCR催化活性的影响[82] Fig.5 Effects of hydrothermal aging on NH3-SCR activity of Cu/zeolites [82] |
为改善分子筛催化剂的水热稳定性,DU等[86]通过在Fe-ZSM-5分子筛表面包裹一层硅沸石(silicalite-1),制备具有核壳结构的Fe-ZSM-5@silicalite-1催化剂,该催化剂的水热稳定性和催化活性明显高于Fe-ZSM-5。通过在Mn0.2Ti0.8O2催化剂表面负载SiO2,也能提高催化剂的抗水性能[87]。可见,通过在催化剂表面包裹一层水热稳定性好的材料,有助于提高催化剂的水热稳定性。
4.4 其他中毒机制影响催化剂催化活性的因素众多,研究不同因素对催化剂催化活性的影响,有助于提高催化剂的抗中毒性能。低温条件下,NO2与催化剂表面的NH4+反应会生成NH4NO3,当NH4NO3的生成速率大于消耗速率时,便会积累在催化剂表面,堵塞Cu-SSZ-13催化剂的孔道,降低催化活性[31]。相比BEA分子筛,低温条件下SSZ-13和SAPO-34分子筛表面形成的硝酸铵盐更稳定[88]。开发利用生物柴油,能够缓解石油资源紧缺,但是,生物柴油中含有K、P、Na、Ca、Zn、Mg等元素,需要考虑SCR催化剂对其耐受程度。引入K会减少Fe-BEA催化剂中含铁活性位点,从而降低NH3-SCR催化活性[89],KCl会影响MnOx上的有效氧的数量和迁移能力,从而降低催化剂的催化活性[90];P中毒的CeO2-MoO3/TiO2催化剂表面会形成磷酸盐,覆盖在活性金属位点上,从而降低催化剂的催化活性[91];当Cu-SSZ-13催化剂中存在Na+时,高温条件下,Na+离子会弱化铜离子与分子筛骨架的键能,使其形成CuOx,从而降低催化剂的水热稳定性和高温催化活性[26];Ca会减少CeO2-WO3催化剂表面的酸性位和NH3吸附量,抑制催化剂的催化活性[92]。可见,催化剂中毒的两种主要形式:一是表面催化活性点位被覆盖,致使催化反应过程无法进行;二是催化活性组分与有害组分结合生成不具备催化活性的组分,从而破坏催化剂结构,使催化剂失活。
5 催化反应动力学NH3-SCR气固催化反应为多相催化反应,分析该催化反应过程,建立催化反应数学模型,能够直观地表达不同过程因素对结果的影响[93]。FAHAMI等[94]研究了Cu-CHA型催化剂催化氧化NO的反应速率模型,并推导出了NO氧化速率表达式。HU等[95]研究了孔扩散对Cu/SAPO-34催化剂NH3-SCR催化反应的本征动力学的影响,并得出表观反应活化能的值为44.8 ±3.0 kJ·mol-1。OLSSON等[96]以Cu/SSZ-13为研究对象,建立了硫中毒和硫中毒催化剂再生模型,描述了催化剂硫中毒过程和硫中毒催化剂再生过程。SHWAN等[97]建立了预测Fe-BEA催化剂水热失活、磷中毒、钾中毒的复合反应动力学模型。SUPRIYANTO等[98]研究了铜基分子筛催化剂水热失活模型,该模型考察了水热处理对NH3吸脱附、NH3氧化、NO氧化、标准NH3-SCR反应和快速NH3-SCR反应的影响。上述催化反应动力学模型的建立,有利于帮助预测实际反应条件对催化剂催化活性的影响,从而为设计催化反应系统提供理论依据。
6 结论与展望铁氧化物、锰氧化物、铈氧化物和分子筛催化剂在实验条件下均具有良好的NH3-SCR催化活性,为了更好地将其运用于处理实际柴油车尾气,氧化物催化剂的催化活性温度窗口还有待拓宽,分子筛催化剂的水热稳定性还需要改善。不同催化剂的构效关系、催化反应机理和中毒机制还需进一步明确。为了降低尾气处理成本、提高处理效率,将LNTs技术和SCR技术结合运用,既有效消除LNTs技术生成的NH3,又无需额外通过尿素热解提供SCR的还原剂NH3。柴油车除需要控制NOx排放外,还需要控制颗粒物的排放。将SCR装置与DPF结合,开发SDPF技术(SCR耦合DPF技术),可缩小尾气净化装置体积、降低成本。可见,将SCR技术与其他尾气处理技术结合,开发新型、高效的耦合技术是今后的研发方向。
| [1] |
FORZATTI P. Present status and perspectives in de-NOx SCR catalysis[J]. Applied Catalysis A: General, 2001, 222(1-2): 221-236. DOI:10.1016/S0926-860X(01)00832-8 |
| [2] |
TWIGG M V. Catalytic control of emissions from cars[J]. Catalysis Today, 2011, 163(1): 33-41. DOI:10.1016/j.cattod.2010.12.044 |
| [3] |
施赟. Cex/HBEA催化剂选择性催化还原NOx机制及其抗硫抗积炭特性[D].杭州: 浙江大学, 2017. SHI Y. The study on Cex/HBEA catalyst for selective catalytic reduction of NOx and its resistance to SO2 and C3H6[D]. Hangzhou: Zhejiang University, 2017. http://cdmd.cnki.com.cn/Article/CDMD-10335-1017071506.htm |
| [4] |
BUSCA G, LIETTI L, RAMIS G, et al. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: a review[J]. Applied Catalysis B: Environmental, 1998, 18(1-2): 1-36. DOI:10.1016/S0926-3373(98)00040-X |
| [5] |
DUNN J P, KOPPULA P R, STENGER H G, et al. Oxidation of sulfur dioxide to sulfur trioxide over supported vanadia catalysts[J]. Applied Catalysis B: Environmental, 1998, 19(2): 103-117. DOI:10.1016/S0926-3373(98)00060-5 |
| [6] |
OKAZAKI S, KUROHA H, OKUYAMA T. Effect of Nb2O5 addition on the catalytic activity of feox for reduction of NOx with NH3 and O2[J]. Chemistry Letters, 1985, 14(1): 45-48. |
| [7] |
LI X, LI J H, PENG Y, et al. Selective catalytic reduction of NO with NH3 over novel iron-tungsten mixed oxide catalyst in a broad temperature range[J]. Catalysis Science & Technology, 2015, 5(9): 4556-4564. |
| [8] |
SUN P, GUO R T, LIU S M, et al. The enhanced performance of MnOx catalyst for NH3-SCR reaction by the modification with Eu[J]. Applied Catalysis A: general, 2017, 531: 129-138. DOI:10.1016/j.apcata.2016.10.027 |
| [9] |
FANG N J, GUO J X, SHU S, et al. Enhancement of low-temperature activity and sulfur resistance of Fe0.3Mn0.5Zr0.2 catalyst for NO removal by NH3-SCR[J]. Chemical Engineering Journal, 2017, 325: 114-123. DOI:10.1016/j.cej.2017.05.053 |
| [10] |
FANG D, HE F, LIU X Q, et al. Low temperature NH3-SCR of NO over an unexpected Mn-based catalyst: Promotional effect of Mg doping[J]. Applied Surface Science, 2018, 427: 45-55. DOI:10.1016/j.apsusc.2017.08.088 |
| [11] |
CHEN H F, XIA Y, HUANG H, et al. Highly dispersed surface active species of Mn/Ce/TiW catalysts for high performance at low temperature NH3-SCR[J]. Chemical Engineering Journal, 2017, 330: 1195-1202. DOI:10.1016/j.cej.2017.08.069 |
| [12] |
YAO X J, KONG T T, YU S H, et al. Influence of different supports on the physicochemical properties and denitration performance of the supported Mn-based catalysts for NH3-SCR at low temperature[J]. Applied Surface Science, 2017, 402: 208-217. DOI:10.1016/j.apsusc.2017.01.081 |
| [13] |
LI X L, LI Y H. Selective catalytic reduction of NO with NH3 over Ce-Mo-Ox Catalyst[J]. Catalysis Letters, 2014, 144(1): 165-171. DOI:10.1007/s10562-013-1103-6 |
| [14] |
LIU K, LIU F D, XIE L J, et al. DRIFTS study of a Ce-W mixed oxide catalyst for the selective catalytic reduction of NOx with NH3[J]. Catalysis Science & Technology, 2015, 5(4): 2290-2299. |
| [15] |
ESCH F, FABRIS S, ZHOU L, et al. Electron localization determines defect formation on ceria substrates[J]. Science, 2005, 309(5735): 752-755. DOI:10.1126/science.1111568 |
| [16] |
GUO R T, ZHEN W L, PAN W G, et al. Effect of Cu doping on the SCR activity of CeO2 catalyst prepared by citric acid method[J]. Journal of Industrial and Engineering Chemistry, 2014, 20(4): 1577-1580. DOI:10.1016/j.jiec.2013.07.051 |
| [17] |
WANG S, ZHENG M H, LI M, et al. Synergistic effects towards H2 oxidation on the Cu-CeO2 electrode: a combination study with DFT calculations and experiments[J]. Journal of Materials Chemistry A, 2016, 4(15): 5745-5754. DOI:10.1039/C6TA00732E |
| [18] |
ZHANG L, ZOU W X, MA K L, et al. Sulfated temperature effects on the catalytic activity of CeO2 in NH3-selective catalytic reduction conditions[J]. The Journal of Physical Chemistry C, 2015, 119(2): 1155-1163. DOI:10.1021/jp511282c |
| [19] |
WEI L, CUI S P, GUO H X, et al. DRIFT and DFT study of cerium addition on SO2 of manganese-based catalysts for low temperature SCR[J]. Journal of Molecular Catalysis A Chemical, 2016, 421: 102-108. DOI:10.1016/j.molcata.2016.05.013 |
| [20] |
IWAMOTO M, FURUKAWA H, MINE Y, et al. Copper(Ⅱ) ion-exchanged ZSM-5 zeolites as highly active catalysts for direct and continuous decomposition of nitrogen monoxide[J]. Journal of the Chemical Society Chemical Communications, 1986(16): 1272-1273. DOI:10.1039/c39860001272 |
| [21] |
SATO S, YOSHIHIRO Y, YAHIRO H, et al. Cu-ZSM-5 zeolite as highly active catalyst for removal of nitrogen monoxide from emission of diesel engines[J]. Applied Catalysis, 1991, 70(1): L1-L5. |
| [22] |
BRANDENBERGER S, KR CHER O, TISSLER A, et al. The state of the art in selective catalytic reduction of NOx by ammonia using metal-exchanged zeolite catalysts[J]. Catalysis Reviews, 2008, 50(4): 492-531. DOI:10.1080/01614940802480122 |
| [23] |
BARAN R, AVERSENG F, WIERZBICKI D, et al. Effect of postsynthesis preparation procedure on the state of copper in CuBEA zeolites and its catalytic properties in SCR of NO with NH3[J]. Applied Catalysis A: General, 2016, 523: 332-342. DOI:10.1016/j.apcata.2016.06.008 |
| [24] |
KWAK J H, TONKYN R G, KIM D H, et al. Excellent activity and selectivity of Cu-SSZ-13 in the selective catalytic reduction of NOx with NH3[J]. Journal of Catalysis, 2010, 275(2): 187-190. DOI:10.1016/j.jcat.2010.07.031 |
| [25] |
FICKEL D W, D'ADDIO E, LAUTERBACH J A, et al. The ammonia selective catalytic reduction activity of copper-exchanged small-pore zeolites[J]. Applied Catalysis B: Environmental, 2011, 102(3-4): 441-448. DOI:10.1016/j.apcatb.2010.12.022 |
| [26] |
ZHAO Z C, YU R, ZHAO R R, et al. Cu-exchanged Al-rich SSZ-13 zeolite from organotemplate-free synthesis as NH3-SCR catalyst: effects of Na+ ions on the activity and hydrothermal stability[J]. Applied Catalysis B: Environmental, 2017, 217: 421-428. DOI:10.1016/j.apcatb.2017.06.013 |
| [27] |
IWASAKI M, YAMAZAKI K, BANNO K, et al. Characterization of Fe/ZSM-5 DeNOx catalysts prepared by different methods: relationships between active Fe sites and NH3-SCR performance[J]. Journal of Catalysis, 2008, 260(2): 205-216. DOI:10.1016/j.jcat.2008.10.009 |
| [28] |
FREY A M, MERT S, DUE-HANSEN J, et al. Fe-BEA Zeolite catalysts for NH3-SCR of NO[J]. Catalysis Letters, 2009, 130(1-2): 1-8. DOI:10.1007/s10562-009-9894-1 |
| [29] |
SHI Y, WANG X X, XIA Y F, et al. Promotional effect of CeO2 on the propene poisoning resistance of HBEA zeolite catalyst for NH3-SCR of NOx[J]. Molecular Catalysis, 2017, 433: 265-273. DOI:10.1016/j.mcat.2017.02.020 |
| [30] |
ZHAO Y Y, CHOI B, KIM D. Effects of Ce and Nb additives on the de-NOx performance of SCR/CDPF system based on Cu-BEA zeolite for diesel vehicles[J]. Chemical Engineering Science, 2017, 164: 258-269. DOI:10.1016/j.ces.2017.02.009 |
| [31] |
LIU Q L, FU Z C, MA L, et al. MnOx-CeO2 supported on Cu-SSZ-13: a novel SCR catalyst in a wide temperature range[J]. Applied Catalysis A: General, 2017, 547: 146-154. DOI:10.1016/j.apcata.2017.08.024 |
| [32] |
CHI B, QU H X, XING X, et al. Assembly of hollow CeO2@Fe-ZSM-5 and SCR performance[J]. Journal of Alloys and Compounds, 2017, 726: 906-912. DOI:10.1016/j.jallcom.2017.08.075 |
| [33] |
ZHANG L, DU T Y, QU H X, et al. Synthesis of Fe-ZSM-5@Ce/mesoporous-silica and its enhanced activity by sequential reaction process for NH3-SCR[J]. Chemical Engineering Journal, 2017, 313: 702-710. DOI:10.1016/j.cej.2016.12.108 |
| [34] |
LI Y H, SONG W Y, LIU J, et al. The protection of CeO2 thin film on Cu-SAPO-18 catalyst for highly stable catalytic NH3-SCR performance[J]. Chemical Engineering Journal, 2017, 330: 926-935. DOI:10.1016/j.cej.2017.08.025 |
| [35] |
YANG W, ZHANG R D, CHEN B H, et al. New aspects on the mechanism of C3H6 selective catalytic reduction of NO in the presence of O2 over LaFe1-x(Cu, Pd)xO3-δ perovskites[J]. Environmental Science & Technology, 2012, 46(20): 11280-11288. |
| [36] |
GHASEMIAN N, FALAMAKI C, KALBASI M. Clinoptilolite zeolite as a potential catalyst for propane-SCR-NOx: performance investigation and kinetic analysis[J]. Chemical Engineering Journal, 2014, 236: 464-470. DOI:10.1016/j.cej.2013.10.061 |
| [37] |
SHI Y, TAN S, LI S J, et al. Inhibitory effect of SO2 on side reactions of NH3-SCR over olivine[J]. Catalysis Science & Technology, 2015, 5(7): 3613-3623. |
| [38] |
SAMOJEDEN B, GRZYBEK T. The influence of the promotion of N-modified activated carbon with iron on NO removal by NH3-SCR (Selective catalytic reduction)[J]. Energy, 2016, 116(SI): 1484-1491. |
| [39] |
GUO Q Q, JING W, HOU Y Q, et al. On the nature of oxygen groups for NH3-SCR of NO over carbon at low temperatures[J]. Chemical Engineering Journal, 2015, 270: 41-49. DOI:10.1016/j.cej.2015.01.086 |
| [40] |
LI S J, WANG X X, TAN S, et al. CrO3 supported on sargassum-based activated carbon as low temperature catalysts for the selective catalytic reduction of NO with NH3[J]. Fuel, 2017, 191: 511-517. DOI:10.1016/j.fuel.2016.11.095 |
| [41] |
LIU F D, HE H, ZHANG C B, et al. Mechanism of the selective catalytic reduction of NOx with NH3 over environmental-friendly iron titanate catalyst[J]. Catalysis Today, 2011, 175(1): 18-25. DOI:10.1016/j.cattod.2011.02.049 |
| [42] |
YANG S J, LIAO Y, XIONG S C, et al. N2 selectivity of NO Reduction by NH3 over MnOx–CeO2: Mechanism and Key Factors[J]. The Journal of Physical Chemistry C, 2014, 118(37): 21500-21508. DOI:10.1021/jp5062489 |
| [43] |
HU P P, HUANG Z W, HUA W M, et al. Effect of H2O on catalytic performance of manganese oxides in NO reduction by NH3[J]. Applied Catalysis A: General, 2012, 437: 139-148. |
| [44] |
WANG X X, SHI Y, LI S J, et al. Promotional synergistic effect of Cu and Nb doping on a novel Cu/Ti-Nb ternary oxide catalyst for the selective catalytic reduction of NOx with NH3[J]. Applied Catalysis B: Environmental, 2018, 220: 234-250. DOI:10.1016/j.apcatb.2017.08.021 |
| [45] |
INOMATA M, MIYAMOTO A, UI T, et al. Activities of vanadium pentoxide/titanium dioxide and vanadium pentoxide/aluminum oxide catalysts for the reaction of nitric oxide and ammonia in the presence of oxygen[J]. Industrial & Engineering Chemistry Product Research and Development, 1982, 21(3): 424-428. |
| [46] |
RAMIS G, BUSCA G, BREGANI F, et al. Fourier transform-infrared study of the adsorption and coadsorption of nitric oxide, nitrogen dioxide and ammonia on vanadia-titania and mechanism of selective catalytic reduction[J]. Applied Catalysis, 1990, 64: 259-278. DOI:10.1016/S0166-9834(00)81565-1 |
| [47] |
WANG D, ZHANG L, KAMASAMUDRAM K, et al. In situ-DRIFTS study of selective catalytic reduction of NOx by NH3 over Cu-exchanged SAPO-34[J]. Acs Catalysis, 2013, 3(5): 871. DOI:10.1021/cs300843k |
| [48] |
KLUKOWSKI D, BALLE P, GEIGER B, et al. On the mechanism of the SCR reaction on Fe/HBEA zeolite[J]. Applied Catalysis B: Environmental, 2009, 93: 185-193. DOI:10.1016/j.apcatb.2009.09.028 |
| [49] |
NAM K B, KWON D W, HONG S C. DRIFT study on promotion effects of tungsten-modified Mn/Ce/Ti catalysts for the SCR reaction at low-temperature[J]. Applied Catalysis A: General, 2017, 542: 55-62. DOI:10.1016/j.apcata.2017.05.017 |
| [50] |
MA Z R, WU X D, H RELIND H, et al. NH3-SCR reaction mechanisms of NbOx/Ce0.75Zr0.25O2 catalyst: DRIFTS and kinetics studies[J]. Journal of Molecular Catalysis A: Chemical, 2016, 423: 172-180. DOI:10.1016/j.molcata.2016.06.023 |
| [51] |
PENG Y, LI K Z, LI J H. Identification of the active sites on CeO2–WO3 catalysts for SCR of NOx with NH3: An in situ IR and Raman spectroscopy study[J]. Applied Catalysis B: Environmental, 2013, 140: 483-492. |
| [52] |
BOROŃ P, CHMIELARZ L, GIL B, et al. Experimental evidence of NO SCR mechanism in the presence of the BEA zeolite with framework and extra-framework cobalt species[J]. Applied Catalysis B: Environmental, 2016, 198: 457-470. DOI:10.1016/j.apcatb.2016.06.012 |
| [53] |
CORT S-REYES M, FINOCCHIO E, HERRERA C, et al. A study of Cu-SAPO-34 catalysts for SCR of NOx by ammonia[J]. Microporous and Mesoporous Materials, 2017, 241: 258-265. DOI:10.1016/j.micromeso.2016.11.032 |
| [54] |
YE Q, WANG L F, YANG R T. Activity, propene poisoning resistance and hydrothermal stability of copper exchanged chabazite-like zeolite catalysts for SCR of NO with ammonia in comparison to Cu/ZSM-5[J]. Applied Catalysis A: General, 2012, 427: 24-34. |
| [55] |
MA L, SU W K, LI Z G, et al. Mechanism of propene poisoning on Cu-SSZ-13 catalysts for SCR of NOx with NH3[J]. Catalysis Today, 2015, 245: 16-21. DOI:10.1016/j.cattod.2014.05.027 |
| [56] |
贺泓, 翁端, 资新运. 柴油车尾气排放污染控制技术综述[J]. 环境科学, 2007, 28(6): 1169-1177. HE H, WENG D, ZI X Y. Diesel emission control technologies: a review[J]. Environmental science, 2007, 28(6): 1169-1177. DOI:10.3321/j.issn:0250-3301.2007.06.001 |
| [57] |
MA L, LI J H, CHENG Y S, et al. Propene poisoning on three typical Fe-zeolites for SCR of NOx with NH3: From mechanism study to coating modified architecture[J]. Environmental Science & Technology, 2012, 46(3): 1747-1754. |
| [58] |
CAO Y. Cerium promotion on the hydrocarbon resistance of a Cu-SAPO-34 NH3-SCR monolith catalyst[J]. Catalysis Science & Technology, 2015, 5(9): 4511-4521. |
| [59] |
ZHANG T, QIU F, LI J H. Design and synthesis of core-shell structured meso-Cu-SSZ-13@mesoporous aluminosilicate catalyst for SCR of NOx with NH3: enhancement of activity, hydrothermal stability and propene poisoning resistance[J]. Applied Catalysis B: Environmental, 2016, 195: 48-58. DOI:10.1016/j.apcatb.2016.04.058 |
| [60] |
XI Y Z, OTTINGER N A, LIU Z G. New insights into sulfur poisoning on a vanadia SCR catalyst under simulated diesel engine operating conditions[J]. Applied Catalysis B: Environmental, 2014, 160: 1-9. |
| [61] |
ZHANG L, WANG D, LIU Y, et al. SO2 poisoning impact on the NH3-SCR reaction over a commercial Cu-SAPO-34 SCR catalyst[J]. Applied Catalysis B: Environmental, 2014, 156: 371-377. |
| [62] |
OLSSON L, WIJAYANTI K, LEISTNER K, et al. A kinetic model for sulfur poisoning and regeneration of Cu/SSZ-13 used for NH3-SCR[J]. Applied Catalysis B: Environmental, 2016, 183: 394-406. DOI:10.1016/j.apcatb.2015.11.001 |
| [63] |
XU T F, WU X D, GAO Y X, et al. Comparative study on sulfur poisoning of V2O5-Sb2O3/TiO2 and V2O5-WO3/TiO2 monolithic catalysts for low-temperature NH3-SCR[J]. Catalysis Communications, 2017, 93: 33-36. DOI:10.1016/j.catcom.2017.01.021 |
| [64] |
JIN R B, LIU Y, WANG Y, et al. The role of cerium in the improved SO2 tolerance for NO reduction with NH3 over Mn-Ce/TiO2 catalyst at low temperature[J]. Applied Catalysis B: Environmental, 2014, 148: 582-588. |
| [65] |
BROOKSHEAR D W, NAM J G, KE N, et al. Impact of sulfation and desulfation on NOx reduction using Cu-chabazite SCR catalysts[J]. Catalysis Today, 2015, 258: 359-366. DOI:10.1016/j.cattod.2015.04.029 |
| [66] |
WANG C, WANG J, WANG J, et al. The effect of sulfate species on the activity of NH3-SCR over Cu/SAPO-34[J]. Applied Catalysis B: Environmental, 2017, 204: 239-249. DOI:10.1016/j.apcatb.2016.11.033 |
| [67] |
WIJAYANTI K, ANDONOVA S, KUMAR A, et al. Impact of sulfur oxide on NH3-SCR over Cu-SAPO-34[J]. Applied Catalysis B: Environmental, 2015, 166: 568-579. |
| [68] |
SHI Y, TAN S, WANG X X, et al. Regeneration of sulfur-poisoned CeO2 catalyst for NH3-SCR of NOx[J]. Catalysis Communications, 2016, 86: 67-71. DOI:10.1016/j.catcom.2016.08.004 |
| [69] |
FRANCE L J, YANG Q, LI W, et al. Ceria modified FeMnOx-enhanced performance and sulphur resistance for low-temperature SCR of NOx[J]. Applied Catalysis B: Environmental, 2017, 206: 203-215. DOI:10.1016/j.apcatb.2017.01.019 |
| [70] |
KUMAR A, SMITH M A, KAMASAMUDRAM K, et al. Chemical deSOx: an effective way to recover Cu-zeolite SCR catalysts from sulfur poisoning[J]. Catalysis Today, 2016, 267: 10-16. DOI:10.1016/j.cattod.2016.01.033 |
| [71] |
KIM Y J, KWON H J, HEO I, et al. Mn-Fe/ZSM-5 as a low-temperature SCR catalyst to remove NOx from diesel engine exhaust[J]. Applied Catalysis B: Environmental, 2012, 126: 9-21. DOI:10.1016/j.apcatb.2012.06.010 |
| [72] |
MADIA G, ELSENER M, KOEBEL M, et al. Thermal stability of vanadia-tungsta-titania catalysts in the SCR process[J]. Applied Catalysis B: Environmental, 2002, 39: 181-190. DOI:10.1016/S0926-3373(02)00099-1 |
| [73] |
CHAPMAN D M. Behavior of titania-supported vanadia and tungsta SCR catalysts at high temperatures in reactant streams: tungsten and vanadium oxide and hydroxide vapor pressure reduction by surficial stabilization[J]. Applied Catalysis A: General, 2011, 392: 143-150. DOI:10.1016/j.apcata.2010.11.005 |
| [74] |
KHARAS K C C, ROBOTA H J, LIU D J. Deactivation in Cu-ZSM-5 lean-burn catalysts[J]. Applied Catalysis B: Environmental, 1993, 2: 225-237. DOI:10.1016/0926-3373(93)80050-N |
| [75] |
MATSUMOTO S, YOKOTA K, DOI H, et al. Research on new DeNOx catalysts for automotive engines[J]. Catalysis Today, 1994, 22: 127-146. DOI:10.1016/0920-5861(94)80097-9 |
| [76] |
BRANDENBERGER S, KR CHER O, CASAPU M, et al. Hydrothermal deactivation of Fe-ZSM-5 catalysts for the selective catalytic reduction of NO with NH3[J]. Applied Catalysis B: Environmental, 2011, 101: 649-659. DOI:10.1016/j.apcatb.2010.11.006 |
| [77] |
WILKEN N, WIJAYANTI K, KAMASAMUDRAM K, et al. Mechanistic investigation of hydrothermal aging of Cu-BEA for ammonia SCR[J]. Applied Catalysis B: Environmental, 2012, 111: 58-66. |
| [78] |
PARK J H, PARK H J, BAIK J H, et al. Hydrothermal stability of CuZSM5 catalyst in reducing NO by NH3 for the urea selective catalytic reduction process[J]. Journal of Catalysis, 2006, 240(1): 47-57. DOI:10.1016/j.jcat.2006.03.001 |
| [79] |
GRINSTED R A, JEN H W, MONTREUIL C N, et al. The relation between deactivation of CuZSM-5 in the selective reduction of NO and dealumination of the zeolite[J]. Zeolites, 1993, 13(8): 602-606. DOI:10.1016/0144-2449(93)90130-U |
| [80] |
LEISTNER K, OLSSON L. Deactivation of Cu/SAPO-34 during low-temperature NH3-SCR[J]. Applied Catalysis B: Environmental, 2015, 165: 192-199. DOI:10.1016/j.apcatb.2014.09.067 |
| [81] |
XIE K P, WOO J, BERNIN D, et al. Insights into hydrothermal aging of phosphorus-poisoned Cu-SSZ-13 for NH3-SCR[J]. Applied Catalysis B: Environmental, 2019, 241: 205-216. DOI:10.1016/j.apcatb.2018.08.082 |
| [82] |
KWAK J H, TRAN D, BURTON S D, et al. Effects of hydrothermal aging on NH3-SCR reaction over Cu/zeolites[J]. Journal of Catalysis, 2012, 287: 203-209. DOI:10.1016/j.jcat.2011.12.025 |
| [83] |
LIU X S, WU X D, WENG D, et al. Evolution of copper species on Cu/SAPO-34 SCR catalysts upon hydrothermal aging[J]. Catalysis Today, 2017, 281: 596-604. DOI:10.1016/j.cattod.2016.05.021 |
| [84] |
TANG J J, XU M H, YU T, et al. Catalytic deactivation mechanism research over Cu/SAPO-34 catalysts for NH3-SCR (Ⅱ): The impact of copper loading[J]. Chemical Engineering Science, 2017, 168: 414-422. DOI:10.1016/j.ces.2017.04.053 |
| [85] |
LEISTNER K, KUMAR A, KAMASAMUDRAM K, et al. Mechanistic study of hydrothermally aged Cu/SSZ-13 catalysts for ammonia-SCR[J]. Catalysis Today, 2018, 307(SI): 55-64. |
| [86] |
DU T Y, QU H X, LIU Q, et al. Synthesis, activity and hydrophobicity of Fe-ZSM-5@silicalite-1 for NH3-SCR[J]. Chemical Engineering Journal, 2015, 262: 1199-1207. DOI:10.1016/j.cej.2014.09.119 |
| [87] |
YU S H, JIANG N X, ZOU W X, et al. A general and inherent strategy to improve the water tolerance of low temperature NH3-SCR catalysts via trace SiO2 deposition[J]. Catalysis Communications, 2016, 84: 75-79. DOI:10.1016/j.catcom.2016.06.001 |
| [88] |
LEISTNER K, MIHAI O, WIJAYANTI K, et al. Comparison of Cu/BEA, Cu/SSZ-13 and Cu/SAPO-34 for ammonia-SCR reactions[J]. Catalysis Today, 2015, 258: 49-55. DOI:10.1016/j.cattod.2015.04.004 |
| [89] |
SHWAN S, JANSSON J, OLSSON L, et al. Chemical deactivation of H-BEA and Fe-BEA as NH3-SCR catalysts-effect of potassium[J]. Applied Catalysis B: Environmental, 2015, 166: 277-286. |
| [90] |
CIMINO S, LISI L, TORTORELLI M. Low temperature SCR on supported MnOx catalysts for marine exhaust gas cleaning: effect of KCl poisoning[J]. Chemical Engineering Journal, 2016, 283: 223-230. DOI:10.1016/j.cej.2015.07.033 |
| [91] |
YOU Y C, CHANG H Z, ZHU T, et al. The poisoning effects of phosphorus on CeO2-MoO3/TiO2 DeNOx catalysts: NH3-SCR activity and the formation of N2O[J]. Molecular Catalysis, 2017, 439: 15-24. DOI:10.1016/j.mcat.2017.06.013 |
| [92] |
LI X, LI X S, LI J H, et al. High calcium resistance of CeO2-WO3 SCR catalysts: structure investigation and deactivation analysis[J]. Chemical Engineering Journal, 2017, 317: 70-79. DOI:10.1016/j.cej.2017.02.027 |
| [93] |
刘庆航, 晏乃强, 瞿赞, 等. 溴掺杂钒钛催化剂SCR反应动力学研究[J]. 高校化学工程学报, 2017, 31(5): 1193-1200. LIU Q H, YAN N Q, QU Z, et al. Kinetic study on selective catalytic reduction of NOx by Br-doped V2O5/TiO2 catalyst[J]. Journal of Chemical Engineering of Chinese Universities, 2017, 31(5): 1193-1200. DOI:10.3969/j.issn.1003-9015.2017.05.024 |
| [94] |
FAHAMI A R, NOVA I, TRONCONI E. A kinetic modeling study of NO oxidation over a commercial Cu-CHA SCR catalyst for diesel exhaust aftertreatment[J]. Catalysis Today, 2017, 297(SI): 10-16. |
| [95] |
HU X Q, YANG M, FAN D Q, et al. The role of pore diffusion in determining NH3 SCR active sites over Cu/SAPO-34 catalysts[J]. Journal of Catalysis, 2016, 341: 55-61. DOI:10.1016/j.jcat.2016.05.022 |
| [96] |
OLSSON L, WIJAYANTI K, LEISTNER K, et al. A kinetic model for sulfur poisoning and regeneration of Cu/SSZ-13 used for NH3-SCR[J]. Applied Catalysis B: Environmental, 2016, 183: 394-406. DOI:10.1016/j.apcatb.2015.11.001 |
| [97] |
SHWAN S, JANSSON J, OLSSON L, et al. Deactivation mechanisms of iron-exchanged zeolites for NH3-SCR applications[J]. Catalysis Today, 2015, 258: 432-440. DOI:10.1016/j.cattod.2015.01.003 |
| [98] |
SUPRIYANTO, WIJAYANTI K, KUMAR A, et al. Global kinetic modeling of hydrothermal aging of NH3-SCR over Cu-zeolites[J]. Applied Catalysis B: Environmental, 2015, 163: 382-392. DOI:10.1016/j.apcatb.2014.07.059 |




