2. 浙江大学衢州研究院, 浙江 衢州 324000;
3. 浙江恒逸石化有限公司, 浙江 杭州 311209
2. Institute of Zhejiang University-Quzhou, Quzhou 324000, China;
3. Zhejiang Hengyi Petrochemicals Co. Ltd., Hangzhou 311209, China
聚酯因性能优异而成为一类被广泛使用的工程塑料。聚酯合成最早可追溯到1847年,Berzelius使用甘油和酒石酸合成了聚酯[1]。1941年出现了首件聚对苯二甲酸乙二醇酯(PET)专利[2-3],后续开发了PET纤维。经过80多年的发展,聚酯合成工艺和技术日趋成熟。在常见的聚酯中,PET商业化程度最高,其纤维的商品名为涤纶。随着人们生活中塑料用量的剧增,给环境造成了严重的“白色污染”[4],为此,先后出现了以聚对苯二甲酸己二酸丁二醇酯(PBAT)[5]、聚乳酸(PLA)[6]等为代表的可降解聚酯。聚酯合成一般有2种途径,一是缩聚反应,另一种为开环聚合(ROP)。前者通常经历酯化和缩聚2个阶段。在酯化阶段,二酸和二醇经催化发生酯化反应生成酯键,得到低分子量的酯化产物。在随后的缩聚阶段中,使用高温和真空条件,通过酯交换反应将小分子二醇从聚合体系中脱除,最终获得高分子量的聚合物[7]。开环聚合则从内酯或交酯出发,在催化剂作用下单体在引发剂基础上开环增长,得到高分子量的聚酯产品。聚酯合成现阶段主要使用金属催化剂,能够对聚酯合成的酯化和缩聚起催化作用的金属元素很多,几乎遍布所有主、副族元素[8],常用的有锑、钛、锗、锡系催化剂。这些金属催化剂普遍要求聚合底物的杂质含量低,反应需在高温和高真空度下进行,因此,对现有催化剂进行改性以及开发高效、绿色替代品已成为国内外聚酯行业所关注的重点。为此,本文将评述聚酯合成催化剂的研究进展,从锑、钛、锡、锗、铝等金属、非金属和一些其他催化方法出发,介绍相关研发工作的进展,以期为高性能聚酯合成和聚合物性能调控提供指导。
2 金属催化剂 2.1 锑系催化剂锑系催化剂是现阶段商业化聚酯产品中应用最广的催化剂,其活性较高,成本较低,同时,相对于其他金属催化剂副,反应发生更少[9]。常使用的有三氧化二锑(Sb2O3)、醋酸锑(SbAc3)及乙二醇锑(EG3Sb2),早期较多使用前2种催化剂,后者从20世纪90年代开始使用,由于产品性能更优而逐渐成为前两者的替代品[10]。
早在1989年,Yamada[11]就研究了三氧化二锑催化的对苯二甲酸(PTA)和乙二醇(EG)的酯化反应。Toufaili等[12]则系统研究了锑系催化剂在聚酯合成中的催化机理。Papadopoulos等[13]选择Sb2O3和SbAc3,合成了聚2, 5-呋喃二甲酸乙二醇酯(PEF),并根据实验结果建立了反应动力学模型;在190 ℃酯化以及220 ℃缩聚反应中,Sb2O3较SbAc3有更高的催化活性,获得的聚合物具有更高的分子量。Ehsani等[14]比较了Sb2O3、辛酸亚锡(Sn(Oct)2)和氯化亚锡(SnCl2)在乳酸缩聚反应中的催化活性,发现Sb2O3在5 h内即可达到高转换率。
锑催化剂也与其他催化剂复合使用,这样可利用锑系催化剂的高催化活性,减少锑用量,从而降低其对人类和环境的影响。Stanley等[15]将Sb2O3与二氧化锗(GeO2)以及钛酸四异丙酯(TIPT)进行复合,在双金属协同作用下,合成了聚对苯二甲酸乙二醇异山梨醇酯,研究发现Sb2O3的加入可使2种催化剂的反应时间分别从160 min和110 min,缩短至135 min和95 min,而对聚合物分子量几乎不产生影响,其中Sb/Ti催化剂组合的性能最优。在Karayannidis等[16]的研究中,Mg、Mn、Zn、Sn、Ti、Zr、Ge等催化剂应用于聚对苯二甲酸丙二醇酯(PTT)的合成,研究发现在缩聚阶段这些催化剂均可使用Sb2O3作为第二催化剂组分,最终合成了特性黏数[η]为0.5~0.7 dL·g−1的聚酯。
负载型锑催化剂也用于聚酯合成。Ilgen等[17]和Ramadugu等[18]通过理论计算和实验结果,证明Sb和Al之间可形成相互作用,可将Sb负载到高岭土上以提高催化剂性能。Zhang等[19-20]利用EG3Sb2负载到水合氧化铝(主要为γ-AlOOH)上,将得到的催化剂用于合成聚对苯二甲酸间苯二甲酸乙二醇酯(PETI),其催化效率明显高于单独使用EG3Sb2,产物的[η]超过0.75 dL·g−1;当EG3Sb2与γ-AlOOH质量比为60:40时,缩聚速率最高,生成的聚合物数均分子量Mn为23 kg·mol−1,酸值仅13.13 mol·t−1;研究还发现少量的Sb和Al连接到聚合物链末端的羟基上,在结晶过程中形成成核中心,提高了PETI的结晶性,从而使聚合物的熔点和玻璃化转变温度分别从243.2 ℃和74.6 ℃提高到249.2 ℃和78.6 ℃。
虽然锑系催化剂被广泛使用,但锑离子会造成人体的心、肺、肝等组织损伤,严重时还会破坏DNA结构,引发基因毒性或是癌症。生产过程或产品中残留的锑对环境的影响不能忽视,其已被美国环境保护署(USEPA)列入主要污染物清单[21-22]。
2.2 钛系催化剂钛系催化剂是现阶段研究和应用都非常广的催化剂,以钛酸四丁酯(TBT)或TIPT为主。钛系催化剂的催化能力强,所生成的聚合物相对分子质量高,在很多芳香族或脂肪族聚酯合成中可作为锑系催化剂的替代品[10, 23-27],在聚酯生产中得到应用。
Debuissy等[28]研究了1, 4-丁二醇和2, 3-丁二醇与己二酸的缩聚,发现TIPT可高效催化2种聚己二酸丁二醇酯(PBA)的合成,1, 4-丁二醇和2, 3-丁二醇的反应活化能(Ea)分别从不使用催化剂的50.6 kJ·mol−1和83.7 kJ·mol−1,降至37.3 kJ·mol−1和70.7 kJ·mol−1。Terzopoulou等[29]比较了钛系与锡系催化剂在PEF中的催化活性,经2 h缩聚,TBT和TIPT分别制得[η]为0.27 dL·g−1和0.31 dL·g−1的PBA,而使用二丁基氧化锡(DBTO)与Sn(Oct)2,[η]仅能达到0.20和0.17 dL·g−1。Gan等[30]使用TIPT和甲基磺酸催化合成了PET基聚醚酯,用二甘醇或乙二醇低聚物替代PET基聚醚酯中的乙二醇,经200 ℃酯化、260 ℃与20 Pa缩聚5 h,得到[η]最高为1.04 dL·g−1的聚酯,断裂伸长率显著提高至408%。
由于钛酸酯类催化剂活性非常高,且易水解,在聚合过程中易产生副反应,增加产品色泽,因此常用双齿或多齿配体对钛酸酯类催化剂进行改性。图 1为双齿配合的Ti系催化剂[27],配体的螯合作用使其与钛原子紧密结合,从而降低催化活性和副反应程度,还可以进一步通过调整配体的位阻和电子效应来增加钛系催化剂的稳定性[26]。在开环聚合中所使用螯合配体改性的钛系催化剂,都比Sn(Oct)2有更好的催化活性、稳定性和选择性[31-32]。Shigemoto等[33]将四配位含有酚醛基席夫碱的螯合配体改性的钛催化剂用于合成聚己内酯(PCL)和PLA,发现螯合配体改性的催化剂均使缩聚反应的Ea有所提升,从钛酸四乙酯(Ti(OEt)4)的64.7 kJ·mol−1升至99.9 kJ·mol−1。Gao等[34]使用了Salen配体制备八面体形的催化剂,在丙交酯(LA)开环聚合中,LA转化率可达97%。Santoro等[35]将二酚或杯[n](n=4,6,8)芳烃配体改性的钛系催化剂用于环酯的开环聚合,发现配合物几何结构对催化能力及聚合物性能都有极大的影响,其中,带亚甲基桥改性的催化剂在合成PCL的开环聚合中活性最高,甲苯溶液聚合中的单体接近完全转化,PCL的Mn达72 kg·mol−1。Upitak等[36]研究了吡咯醛基的席夫碱配体的六配位钛系催化剂在合成PCL和PLA的开环聚合中的催化能力,发现催化剂均具有良好的催化性能,可合成高分子量、窄分布的聚酯。
在协同催化方面,Mamkhegov等[37]将工业上常用的TBT、Sb2O3等催化剂进行复合,用于合成PET和聚对苯二甲酸丁二醇酯(PBT);当在聚合过程中使用次磷酸钙(Ca(H2PO2)2)和硼酸,催化剂催化能力得到较大提升;通过分析聚合物颜色变化,发现使用TBT-Ca(H2PO2)2-SnCl2催化剂体系可使PBT颜色从暗灰色变成亮白色,聚合物热稳定性也得到提升。Li等[38-39]研究了淀粉基Ti-Mg双金属复合催化剂在聚对苯二甲酸乙二醇异山梨醇酯与PTT合成中的性能,发现复合催化剂较常规催化剂有更好的溶解性和稳定性,聚合过程中存在更少的副反应,聚酯的色泽更浅。
在有机合成中广泛使用的钛硅复合氧化物也常用于聚酯合成。Acordis公司开发了一种商标名为C94的固体缩聚催化剂[40],它是一种二氧化钛/二氧化硅的共沉淀物,可用于合成PET、PBT、PTT等聚酯,活性是锑系催化剂的6~8倍,稳定性高于钛酸酯类催化剂,聚酯产物色泽更浅[41]。Thakur等[42]对钛硅氧化物复合催化剂进行了改性,将长线性烷基、环烷基和芳基等引入催化剂中,提高表面Ti原子位点附近的疏水性,Lewis酸性得到提高,在对苯二甲酸双羟乙酯的缩聚过程中表现出了更好的催化性能。Stȩpień等[43]将这种钛硅复合氧化物用于聚丁二酸丁二醇酯(PBS)的合成,由于催化剂耐水解能力提高,从而使酯化阶段从TBT催化的120 min缩短至60 min。
2.3 锡系催化剂锡系催化剂常用于合成以PLA为代表的脂肪族聚酯。早期人们通过乳酸直接缩聚法合成PLA,催化剂选用强酸条件下的SnCl2[44-46]或氧化亚锡(SnO)[45]等,但需在180~200 ℃、低于600 Pa且长时间反应才能制得高分子量PLA。而ROP则可在130 ℃、常压下较短时间内制得窄分布的高分子量PLA[47-48]。
ROP中常使用以Sn(Oct)2为代表的锡类催化剂。与阳离子和阴离子催化剂相比,锡系催化剂催化的聚合过程中副反应少,合成的聚合物分子量高、分布窄,聚酯链结构更易调控[49-51],因而广泛用于合成PLA[52-53]、聚乙醇酸(PGA)[52, 54-55]、PCL[56]等,也是工业生产中最常用的开环聚合催化剂[57]。Lee等[58]研究了Sn(Oct)2催化PBS合成中回收的副产物环状内酯的开环聚合,所合成的PBS的Mn可达95 kg·mol−1,高于一般熔融缩聚法;动力学研究表明该PBS合成更接近于链增长机理,因而可在较短时间内合成高分子量聚酯。Morales等[59]将该方法用于呋喃二甲酸基聚酯的合成,将半制备层析分离出的环状二聚体、三聚体和四聚体聚酯,用于Sn(Oct)2催化的开环聚合,制得重均分子量Mw为50~60 kg·mol−1的PEF与聚2, 5-呋喃二甲酸丁二醇酯(PBF)。de Jong等[60]发现在制备PEF的固相缩聚中,锡系催化剂较钛系催化剂可制得更高分子量的聚酯。在熔融缩聚制备共聚酯中,同样可使用锡系催化剂。Lee等[61]将Sn(Oct)2用于PBS和PBT的熔融/固相缩聚,合成了多嵌段的聚对苯二甲酸丁二酸丁二醇酯(PBST)共聚酯,在PBS与PBT物质的量比为2:1条件下反应20 min,得到的大分子链中最高的数均嵌段数为7.3。Velmathi等[62]比较了一系列金属催化剂在合成PBS的缩聚中的催化作用,发现在微波的辅助下,SnCl2的催化活性高于TIPT等金属催化剂,在聚丁二酸乙二醇酯(PES)、聚丁二酸丙二醇酯(PPS)以及对应的癸二酸基聚酯合成中也得到相同结论。环酯或者内酯之间的共聚也同样可使用锡系催化剂,Wang等[63-66]将Sn(Oct)2用于ε-己内酯(ε-CL)和LA的开环共聚,定制了聚酯大分子单体,并进行了相关动力学和模型研究。
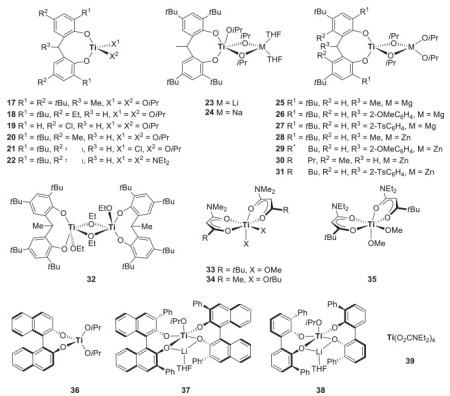
Möller等[67]用配体改性锡酸酯类催化剂,将三氟甲磺酸亚锡(Sn(OTf)2)用于催化β-丁内酯及ε-CL的ROP,发现三氟酸盐取代基增加了锡催化中心的Lewis酸性,使得底物能与醇引发剂进行更有效的交换和均质引发,催化能力优于Sn(Oct)2,反应速率更快,产物分子量与分布更易调控。该催化剂 [68-69]还具有立构选择性,可合成立体规整的PLA。Kricheldorf等[70-73]将芳香官能团取代的有机锡化合物用于PLA的合成,发现其可催化缩聚和开环聚合,从而制得环状PLA。Kricheldorf等[74]还研究了锡酸酯的ROP催化机理(如图 2所示),首先羰基氧与Sn原子的一个空轨道相互作用,使羰基C变得更亲电、Sn─O键更亲核;接着是共价键的重排,使得环状单体插入Sn─O键,进行聚合物链增长。
|
图 2 Sn催化剂的配位-插入机理示意图[74] Fig.2 Schematic diagram of Sn catalyst coordination - insertion mechanism [74] |
Lai等[75]进行了催化剂负载研究,将SnCl2通过浸渍法负载在坡缕土上,将得到的负载型催化剂用于PBS的合成;经160 ℃、1 h的酯化和230 ℃、4 kPa、5 h的缩聚,制得Mn为23 kg·mol−1的产物,而相同条件下均相SnCl2只得到15 kg·mol−1的PBA;将反应后聚合物溶解在氯仿中,通过过滤可将催化剂从产物中分离,回收的催化剂经530 ℃、8 h煅烧后仍具有良好活性。
2.4 锗系催化剂锗系催化剂同样用于聚酯生产,尽管成本高,但较钛系催化剂温和稳定,较锑系催化剂则活性高且对环境和人的毒害小,因此在洁净饮料瓶、无色聚酯纤维原料生产中一般选择锗系催化剂[76-77]。
GeO2是最常见的锗系催化剂,然而由于金属氧化物在聚酯反应体系中的溶解度差导致催化活性较低,制约其在工业上的应用。针对GeO2溶解度差的问题,Jacquel等[78]通过乳酸或乙醇酸与GeO2形成螯合物,大大提高其在PBS合成过程中的催化活性,活性仅略低于TBT,且聚合物非常稳定,200 d储存期内[η]几乎不变。Kricheldorf等[79]研究了三乙基锗酸甲酯(Et3GeOMe)与锗酸四乙酯(Ge(OEt)4)在合成PCL的开环聚合中的催化活性,发现Ge(OEt)4反应活性更高,形成了四臂的星形聚酯,生成的环状低聚物摩尔分数仅为1%,但聚合物分子量分布较宽。Shigemoto等[80]通过理论计算,模拟了Ge(OEt)4在PET合成中的催化活性,并与Ti(OEt)4和锑酸三乙酯(Sb(OEt)3)进行比较,发现四面体结构的Ge(OEt)4由于体积较大,很难形成Ge的中间态四面体结构,Ge中心与酯烷氧基氧原子之间的相互作用弱,但是与酯羰氧的配位作用强,从理论计算上说明锗酸酯通过吸引羰基氧催化缩聚反应。Nishiwaki等[81]使用醋酸锌(Zn(AcO)2)与Ge(OBu)4共同催化合成聚1, 2-丁二酸丙二酯,在200 ℃下制得的聚合物分子量较Ti(OEt)4更高,Mn达45 kg·mol−1;180 ℃下得到的聚酯为纯白色,升至200 ℃则呈浅黄色,而相应的Ti(OEt)4/Zn(AcO)2催化产物则呈暗黄色。
通过配体改性也可提升锗系催化剂的催化活性。Guo等[82]将氯化锗与过量的二(三甲基硅基)氨基钠反应,合成了一种含Ge─N键的六配位金属有机化合物,其在室温下可高效催化LA在四氢呋喃(THF)中的开环聚合,反应5 min达到95% 转化率。Finne等[83]以聚乙二醇(PEG)作为螯合配体改性锗系催化剂,用于合成L-聚乳酸(PLLA);当PEG链接数介于7~46时,产物PLLA中检测不到PEG和Ge连接的特征吸收峰,表明聚合过程符合配位-插入机理,Ge四个基团都参与反应;通过选择适当长度的PEG作配体,可合成PLLA-PEG-PLLA的嵌段共聚物。
2.5 铝系催化剂铝系催化剂具有替代锑、钛等催化剂的潜力,其成本低,对环境和人体的危害小[84],但铝系催化剂对水不稳定是制约其工业应用的瓶颈。
Xiao等[85]研究了乙二醇铝(EG3Al2)在PET合成中的催化活性,并与EG3Sb2和Ti(OEt)4进行了比较;280 ℃下进行缩聚,EG3Al2约30 min可将PET的[η]提高至0.88 dL·g−1,酸值为23 mol·t−1;由于EG3Al2对水的稳定性差,因此只能作为缩聚反应催化剂,在酯化结束后加入。铝系催化剂也可用于催化ROP,Chen等[86]将联吡啶双酚酸铝催化马来酸酐和环氧乙烷衍生物的开环聚合。Andrea等[87]研究了氨基酚配位的铝催化剂在环氧乙烷开环聚合中的应用。Hador等[88]将手性Salen改性的铝配合物用于LA开环聚合,并研究了催化机理,表明催化过程遵循双立体选择控制机制,其中在金属和最后插入的乳酸基团间形成的若干立体异构体中,仅有反式构型存在催化活性。
Wang等[84]以铝基金属有机框架(MOF)材料MIL-53作为催化剂合成PET,由于MIL-53具有良好的水解稳定性,因此酯化时即可加入,可高效催化酯化和缩聚反应,制得[η]为0.75 dL·g−1的PET,聚合过程中几乎不存在副反应。虽然MOF材料的孔径可调,但缩聚反应很难在孔道内部进行,聚合场所在催化剂表面。
利用载体可提高铝系催化剂的耐水解能力与催化性能。工业应用中使用凹凸棒土作为载体,其同时提高了聚酯的抗拉强度和储存模量,加快结晶速度,提高热稳定性。Lin等[89]将负载在凹凸棒土上的水合氧化铝用于合成PET,在1~2.5 h内即可得到[η]高于0.65 dL·g−1的PET,催化剂用量少。
3 非金属催化剂 3.1 有机催化剂使用有机催化剂进行聚酯合成的时间较短,2001年出现了将4-二甲基氨基吡啶用于合成PLA的开环聚合催化的首次报道[90]。有机催化剂通常都是结构简单的小分子,容易购买和保存,对水和氧稳定[90-92]。但其催化活性通常低于金属催化剂,因此多局限于高活性单体的开环聚合[91]。
有机碱类催化剂通常含N或P,利用它们较弱的Lewis碱性进行较为温和的催化。以1, 8-二氮杂二环[5.4.0]十一-7-烯(DBU)、1, 5, 7-三氮杂二环[4.4.0]癸-5-烯(TBD)为代表的氮杂环衍生物被称为“超碱”,是ROP较为高效的催化剂,室温下使用摩尔分数为1% 的催化剂催化LA的ROP,其2 h单体转化率可超过98% [93]。与金属催化剂相比,这类催化剂对水的稳定性好。Dzienia等[94]研究了DBU和TBD催化水引发的PCL开环聚合,结果显示虽然TBD在大部分体系中有更高的催化活性,然而在水的存在下催化活性会下降,而DBU仍保持较高的催化活性,是水引发开环聚合的良好催化剂。磷腈碱类催化剂同样是ROP中高活性催化剂。Dove等[95]发现磷腈碱类催化剂可在低温下进行ROP,−78 ℃下进行外消旋丙交酯聚合仍具有高活性和立体选择性。Ren等[96]研究了一种负载在聚苯乙烯微球上的磷腈催化剂催化的δ-戊内酯的开环聚合,发现其有很高的催化活性,也有良好的回收性能,催化剂循环使用5次,活性无明显下降,单体转化率保持在79%~85%。
有机酸类催化剂种类更广,包括氨基酸、脂肪酸、芳香酸以及一些磺酸和磷酸的衍生物。Susperregui等[97-100]对这些有机酸催化剂进行了研究,发现强酸性物质在催化过程中具有双重作用,它们既是质子的供体,也是质子的受体,因此具有高催化活性。具体而言,三氟甲磺酸和甲磺酸是合成PCL开环聚合的高效催化剂[97-98],可很好地控制开环聚合的进程。三氟甲磺酸在合成PLA的开环聚合中同样具有高活性[101]。Delcroix等[100]研究了二苯基磷酸(DPP)催化的ε-CL的ROP,发现它可在室温下高效催化,转化率超过95%,所得PCL的分子量分布指数Ð仅约1.08。Zhang等[102]考察了方酸(SA)催化的ε-CL的开环聚合中,以苄醇为引发剂,80 ℃下聚合过程呈可控/活性特征,制得窄分布的PCL;同时,SA很容易回收再利用,重复使用10次,催化活性仍无明显降低。Mezzasalma等[103]在LA和ε-CL开环共聚中,选择了苯甲酸作为催化剂;通过不同单体配比等调节,定制了不同结构的共聚酯,并探究了链结构与性能之间的构效关系。Wang等[104]将(±)-联萘酚磷酸酯(rac-BNPH)作为LA与ε-CL开环共聚的催化剂,并与Sn(Oct)2进行了比较,发现rac-BNPH具有高效催化能力,且可避免金属催化剂所造成的聚合物链末端金属基团的残留,从而大幅降低了由于末端金属存在所引起的酯交换,聚合物的分子量和分布更易调控。
3.2 离子液体离子液体虽不含金属元素,但有和金属催化剂相似的性质,具有良好的溶解性和可回收性,是一种有潜力的绿色催化剂。与常规的有机化合物相比,离子液体有无机盐的性质,对水或者离子化合物等的溶解能力较强,同时,其结构中的长链烷烃基团又使其对很多有机物有较高的相容性,因此常作为相转移催化剂,增加反应底物和催化剂间的接触几率,以提高反应速率[105]。
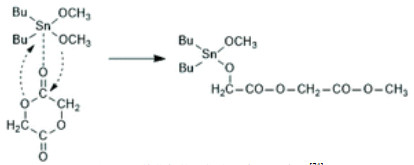
Qu等[106]将咪唑基离子液体应用到PEF的合成中,用不同阴离子[CnMIM]+和阳离子四氟合硼酸根、六氟合磷酸根、双三氟甲磺酰亚胺阴离子等组成的咪唑离子液体,催化合成PEF,产物的Mw高达101 kg·mol−1;与TBT、GeO2、SbAc3及Sn(Oct)2等催化剂进行比较,发现[CnMIM]BF4催化的反应速率仅次于Sn(Oct)2,且合成的PEF分子量和分布与金属催化剂所合成的相当;密度泛函理论研究表明,离子液体催化符合Lewis酸催化机理,其示意图见图 3。Song等[107]使用3-(2-羟基-乙基)-1-甲基咪唑溴化离子液体(HEMIMB)催化LA、邻苯二甲酸酐与环氧化合物的开环聚合,发现HEMIMB在LA开环聚合中有很高活性,含催化剂摩尔分数为1% 的THF溶液体系中,80 ℃反应1.5 h单体转化率超过99%。离子液体种类繁多,且容易修饰。Wei等[108]研究了磺酸基取代的离子液体[BSMIM]+在聚丁二酸己二醇酯合成中的应用,并阐释了催化机理;通过对离子液体结构进行修饰,利用末端的磺酸基团与咪唑2号位的H原子协同催化,可进一步提高聚合速率。
|
图 3 C2mim+在酯化和缩聚中的催化机理[106] Fig.3 Catalytic mechanism of C2mim+ in esterification and polycondensation [106] |
酶是近年来出现的催化剂体系。酶以蛋白质为主,在蛋白质发生多次折叠时可形成非常特别的结构,这种结构大多对底物有较强的识别能力,因此酶催化的反应在区域、化学和立构上均有很高的选择性[8]。选用酶催化合成聚酯,可根据酶的特性选择相应的聚合条件,由于通常条件温和且容易控制,因而可节省能耗。此外,利用酶选择位点的特异性,还可实现聚合物特定位置的改性或修饰,合成出具有特殊性能的聚合物。
脂肪酶用于催化ROP的研究最早出现在1993年,由2个研究小组分别报道使用了Pseudomonas fluorescens lipase与porcine pancreatic lipase 2种脂肪酶[109-110]。其后发展迅速,成为合成高分子量或手性脂肪族聚酯的一种新方法[111]。Comerford等[112]利用Candida antarctica lipase B,在无溶剂的条件下可快速高效合成低分子量的脂肪族和芳香族聚酯,酶可多次回收再利用;这种酶还可用于内酯的开环聚合或缩聚[113]。Schmidt等[114-115]用3种不同的催化酶复合,成功合成了PCL,该方法被称为系统生物催化,其目的是在体外构建人工代谢途径。
4 其他催化技术 4.1 电催化电化学方法可用于聚酯合成,但现阶段研究报道还较少。使用电化学方法,可有效地降低聚合温度,有益于聚合反应的调控。Zhong等[116]用电流激活的Co-Zn催化剂催化O-羧酸酐开环聚合,合成了Ð小于1.1的脂肪族聚酯;研究发现在恒定电流的驱动下,15 min反应单体转化率就超过27%,而一旦停止电流输送,经超过30 min的反应,转化率也仅达到29%,而一旦电流恢复,聚合反应也立即继续发生,并可在1 h内达到82% 转化率。
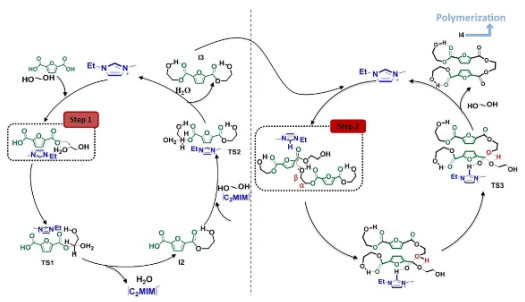
电化学方法还可有效调控催化剂性能。Qi等[117]利用循环电势改变催化剂性能,实现电化学切换调控的开环聚合;在循环电势作用下,铁催化剂的价态在+2和+3之间切换,可分别催化LA和环氧环己烷的开环聚合,最终合成出PLA与聚环氧环己烷的嵌段共聚物(如图 4所示),实现聚合物链结构的有效调控。
|
图 4 循环电势作用下的可切换开环聚合[117] Fig.4 Switchable ring-opening polymerization assisted by a cyclic potential [117] |
利用合适波长的光可对聚合反应进行催化。与传统催化剂相比,光诱导催化的原子利用率高,有时甚至可将反应条件苛刻的过程变得容易实现[118]。光诱导的环加成反应还可定制具有星型、接枝、环状等拓扑结构的聚合物[119-120]。近年来,光催化也被应用到聚酯合成中。Baysak等[121]制备了含有全氟苯酯部分的双蒽基化合物,在365 nm紫外线照射下进行聚合,通过光诱导[4+4]环加成聚合,并与活性酯取代反应相结合,在室温条件下制备了含全氟苯酯侧链的聚酯。光诱导催化也用于环状聚酯的合成。现阶段环状聚酯合成主要有2种途径,一是通过金属有机配合物或N杂环卡宾催化的单体扩环聚合[122-124],另一种是含α, ω-双官能团的线性前驱体的单分子闭环[125]。前者可生产高纯度、高分子量的环状聚合物,但存在分子量和分布难以控制的问题。而后者虽能可控地合成环状聚合物,但很难达到高分子量。通过光诱导催化的电环化或Diels-Alder反应,就可高效可控地合成所需的环状聚酯。Wang等[126]通过光诱导α, ω-蒽基PCL闭环,合成了环大小可调的高分子量环状PCL,合成中仅需在室温下365 nm照射含单体的四氢呋喃溶液体系,即可高效地完成聚合;通过调整溶液中的聚合物浓度,即可实现PCL环尺寸的调节。Josse等[127]基于光诱导异喹诺二甲烷和丙烯酸酯的Diels-Alder反应,合成了高纯度的环聚酯;通过选择合适的引发剂进行交酯或内酯的开环聚合,并对开环聚合产物末端进行适当修饰,即可获得所需的前驱体,再利用光异构化反应,原位获得高活性的双烯结构,进而通过高效的Diels-Alder反应闭环,制得高分子量的环酯;研究人员还将该方法拓展到PLA体系,以说明该合成方法的普适性。
4.3 微波辅助微波是一种频率为0.3~300 GHz的电磁波,其中,2.45 GHz微波已被确定为工业、科学和医疗无线电波段。自20世纪60年代以来,微波被广泛用于干燥木材、固化橡胶和加热食品[128]。在微波加热过程中,微波直接作用于极性分子,从物质内部产生热量[129],比传统加热从外部加热物质、热量通过传导和对流传递到物质内部的方式更高效,使微波辅助化学合成具有反应时间短、收率高、选择性高等优点[130-131]。因此,微波加热方式也可用于聚酯合成。2001年Kéki等[131]报道了使用微波合成D, L-乳酸低聚物(ODLLA),使用家用微波炉进行微波加热,反应20 min所合成的ODLLA的Mn与100 ℃、24 h所得到的聚合物相同。Yamada等[132]对微波在合成PLLA的溶液聚合中所起到的辅助作用进行了定量研究,发现三氟甲磺酸钪催化的二甲苯溶液体系中辐照功率为100~400 W的微波可起到良好的辅助催化作用,300 W下可得到最高的Mw(46.7 kg·mol−1);将电磁波中的电场和磁场分离,研究了单模式微波的作用,发现PLLA分子量随磁场功率增大而减小,随电场功率的增加而增大,表明电场中存在微波的非热效应。Espinosa等[133]考察了微波在PTA和EG的酯化反应中的作用,在不脱除水和EG的条件下,可制备低分子量PET,且由于微波辐射增强了官能团间的碰撞概率,因此缩短了聚合时间。Nagahata等[134]报道了微波对合成PBS中缩聚反应的加速作用,并探究了加速机理;在丁二酸和丁二醇(物质的量比为1:1)的体系中,监测了反应过程中水的脱除,与常规加热相比,发现微波辅助的聚合反应Ea降低了约50%,同时还能激发体系中的水分子,使其更容易从体系中被移除,从而加快了聚合速率。
5 总结与展望现有聚酯合成中虽出现了有机、离子液体、酶等催化剂以及电、光、微波等辅助技术,但金属催化剂仍占主导,缩聚反应中常用锑与钛系催化剂,而开环聚合仍以锡系催化剂为主。这类金属催化剂虽已被广泛应用,但目前大多数催化体系仍存在难以解决的问题:以TBT为代表的金属催化剂难以控制其催化剂活性,会带来催化能力和副反应之间的矛盾;以DBU为代表的有机催化剂大多催化活性较低而仅适用于部分聚酯体系,且往往催化剂用量较大;各种金属催化剂对环境和人体会带来一定危害;绝大多数催化体系缺乏对催化机理及动力学规律的系统、深入的研究。
通过将金属催化剂与其他金属催化剂复合、配体改性以及催化剂负载,可调控催化体系的活性,同时降低副反应,改善产品色泽;进一步对有机催化剂进行改性或配方研究,在其活性和选择性上定向改造;研究各种绿色催化剂(有机催化剂、离子液体催化剂、酶等)在工业领域的应用可能,并逐步取代传统金属催化剂,实现聚酯的绿色催化;开发催化技术改进或开发过程中,离不开对聚合机理和动力学的深入了解,使用先进的原位表征技术如Operando技术将有助于对机理的揭示;动力学的实验结果与模型研究相结合,辅以机器学习等技术,可加速高效催化体系的开发。
催化体系与聚合过程的结合,制约着所合成聚合物的链结构,通过对聚酯链结构与物理、加工、降解等性能之间构效关系的研究,结合对聚合动力学规律的掌握,在指导催化体系高效合成的同时,可实现聚酯产品的高效定制[135]。此外,除了开发新型催化体系,高效催化辅助技术也有待深入研究。通过高效、绿色催化体系的开发,实现聚酯合成技术的提升,促进聚酯工业的进步。
| [1] |
SEYMOUR R B. Polyester mortars[M]. Washington: American Chemical Society, 1979: 61-66.
|
| [2] |
WHINFIELD J R. Chemistry of 'Terylene'[J]. Nature, 1946, 158(4026): 930-931. DOI:10.1038/158930a0 |
| [3] |
WHINFIELD J R, DICKSON J T. Improvements relating to the manufacture of highly polymeric substances[J]. British Patent, 1941, 578: 79. |
| [4] |
朱振林, 王松林, 姜冰雪, 等. 聚酯生物降解及评价方法研究[J]. 化工学报, 2022, 73(1): 110-121. ZHU Z L, WANG S L, JIANG B X, et al. Study on biodegradation of polyesters and their evaluation methods[J]. CIESC Journal, 2022, 73(1): 110-121. |
| [5] |
KANWAL A, ZHANG M, SHARAF F, et al. Polymer pollution and its solutions with special emphasis on poly (butylene adipate terephthalate (PBAT))[J]. Polymer Bulletin, 2022, 79(11): 9303-9330. DOI:10.1007/s00289-021-04065-2 |
| [6] |
KALE G, Auras R, Singh S P, et al. Biodegradability of polylactide bottles in real and simulated composting conditions[J]. Polymer Testing, 2007, 26(8): 1049-1061. DOI:10.1016/j.polymertesting.2007.07.006 |
| [7] |
潘祖仁. 高分子化学[M]. 4版. 北京: 化学工业出版社, 2007. AN Z R. Polymer Chemistry[M]. 4th ed. Beijing: Chemical Industry Press, 2007. |
| [8] |
KOBAYASHI S, UYAMA H, KIMURA S. Enzymatic polymerization[J]. Chemical Reviews, 2001, 101(12): 3793-3818. DOI:10.1021/cr990121l |
| [9] |
BAROT A A, PANCHAL T M, PATEL A, et al. Polyester the workhorse of polymers: A review from synthesis to recycling[J]. Archives of Applied Science Research, 2019, 11(2): 19. |
| [10] |
THOMAS S, RANE A, KANNY K, et al. Recycling of polyethylene terephthalate bottles[M]. Oxford: William Andrew, 2018.
|
| [11] |
YAMADA T. Effect of diantimony trioxide on direct esterification between terephthalic acid and ethylene glycol[J]. Journal of Applied Polymer Science, 1989, 37(7): 1821-1835. DOI:10.1002/app.1989.070370707 |
| [12] |
EL-TOUFAILI F A, FEIX G, REICHERT K H. Mechanistic investigations of antimony-catalyzed polycondensation in the synthesis of poly (ethylene terephthalate)[J]. Journal of Polymer Science Part A: Polymer Chemistry, 2006, 44(3): 1049-1059. DOI:10.1002/pola.21200 |
| [13] |
PAPADOPOULOS L, ZAMBOULIS A, KASMI N, et al. Investigation of the catalytic activity and reaction kinetic modeling of two antimony catalysts in the synthesis of poly (ethylene furanoate)[J]. Green Chemistry, 2021, 23(6): 2507-2524. DOI:10.1039/D0GC04254D |
| [14] |
EHSANI M, KHODABAKHSHI K, ASGARI M. Lactide synthesis optimization: Investigation of the temperature, catalyst and pressure effects[J]. e-Polymers, 2014, 14(5): 353-361. DOI:10.1515/epoly-2014-0055 |
| [15] |
STANLEY N, CHENAL T, DELAUNAY T, et al. Bimetallic catalytic systems based on Sb, Ge and Ti for the synthesis of poly (ethylene terephthalate-co-isosorbide terephthalate)[J]. Polymers, 2017, 9(11): 590. DOI:10.3390/polym9110590 |
| [16] |
KARAYANNIDIS G P, ROUPAKIAS C P, BIKIARIS D N, et al. Study of various catalysts in the synthesis of poly (propylene terephthalate) and mathematical modeling of the esterification reaction[J]. Polymer, 2003, 44(4): 931-942. DOI:10.1016/S0032-3861(02)00875-3 |
| [17] |
ILGEN A G, TRAINOR T P. Sb (Ⅲ) and Sb (Ⅴ) sorption onto Al-rich phases: Hydrous Al oxide and the clay minerals kaolinite KGa-1b and oxidized and reduced nontronite NAu-1[J]. Environmental Science & Technology, 2012, 46(2): 843-851. |
| [18] |
RAMADUGU S K, MASON S E. DFT study of antimony (V) oxyanion adsorption on α-Al2O3 (1102)[J]. The Journal of Physical Chemistry C, 2015, 119(32): 18149-18159. DOI:10.1021/acs.jpcc.5b02061 |
| [19] |
ZHANG F C, KANG H J, BAI Y P, et al. Catalytic property of poly (ethylene terephthalate-co-isophthalate) synthesized with a novel Sb/Al bimetallic compound catalyst[J]. RSC Advances, 2016, 6(72): 67677-67684. DOI:10.1039/C6RA09055A |
| [20] |
ZHANG F C, WANG Q X, WANG L P, et al. Poly-(ethylene terephthalate-co-isophthalate) synthesized via a Sb/Al bimetallic compound catalyst: The effect of the end groups on the properties of polyester[J]. RSC Advances, 2017, 7(35): 21780-21789. DOI:10.1039/C7RA01681F |
| [21] |
郭文景, 张志勇, 符志友, 等. 锑的淡水水质基准及其对我国水质标准的启示[J]. 中国环境科学, 2020, 40(4): 1628-1636. GUO W J, ZHANG Z Y, FU Z Y, et al. Derivation of aquatic life water quality criteria for antimonyin freshwater and its implication for water quality standard in China[J]. China Environmental Science, 2020, 40(4): 1628-1636. |
| [22] |
CARNEADO S, HERNÁNDEZ-NATAREN E, LÓPEZ-SÁNCHEZ J F, et al. Migration of antimony from polyethylene terephthalate used in mineral water bottles[J]. Food Chemistry, 2015, 166: 544-550. DOI:10.1016/j.foodchem.2014.06.041 |
| [23] |
FERREIRA L P, MOREIRA A N, PINTO J C, et al. Synthesis of poly (butylene succinate) using metal catalysts[J]. Polymer Engineering & Science, 2015, 55(8): 1889-1896. |
| [24] |
GARIN M, TIGHZERT L, VROMAN I, et al. The influence of molar mass on rheological and dilute solution properties of poly (butylene succinate)[J]. Journal of Applied Polymer Science, 2014, 131(20): 40887. DOI:10.1002/app.40887 |
| [25] |
THIELE U K. The current status of catalysis and catalyst development for the industrial process of poly (ethylene terephthalate) polycondensation[J]. International Journal of Polymeric Materials and Polymeric Biomaterials, 2001, 50(3/4): 387-394. |
| [26] |
PILATI F, MUNARI A, MANARESI P, et al. Models for the formation of poly (butylene terephthalate): Effect of water on the kinetics of the titanium tetrabutylate-catalysed reactions: 3[J]. Polymer, 1985, 26(11): 1745-1748. DOI:10.1016/0032-3861(85)90297-6 |
| [27] |
LE ROUX E. Recent advances on tailor-made titanium catalysts for biopolymer synthesis[J]. Coordination Chemistry Reviews, 2016, 306: 65-85. DOI:10.1016/j.ccr.2015.06.006 |
| [28] |
DEBUISSY T, POLLET E, AVÉROUS L. Synthesis of potentially biobased copolyesters based on adipic acid and butanediols: Kinetic study between 1, 4-and 2, 3-butanediol and their influence on crystallization and thermal properties[J]. Polymer, 2016, 99: 204-213. DOI:10.1016/j.polymer.2016.07.022 |
| [29] |
TERZOPOULOU Z, KARAKATSIANOPOULOU E, KASMI N, et al. Effect of catalyst type on molecular weight increase and coloration of poly (ethylene furanoate) biobased polyester during melt polycondensation[J]. Polymer Chemistry, 2017, 8(44): 6895-6908. DOI:10.1039/C7PY01171G |
| [30] |
GAN Z Y, QU S, LI S J, et al. Facile synthesis of PET-based poly (ether ester) s with striking physical and mechanical properties[J]. Reactive and Functional Polymers, 2021, 164: 104936. DOI:10.1016/j.reactfunctpolym.2021.104936 |
| [31] |
PÄRSSINEN A, KOHLMAYR M, LESKELÄ M, et al. Catalytic polymerization of ε-caprolactone in air[J]. Polymer Chemistry, 2010, 1(6): 834-836. DOI:10.1039/c0py00107d |
| [32] |
PERI D, MEKER S, MANNA C M, et al. Different ortho and para electronic effects on hydrolysis and cytotoxicity of diamino bis (phenolato) "salan" Ti (Ⅳ) complexes[J]. Inorganic Chemistry, 2011, 50(3): 1030-1038. DOI:10.1021/ic101693v |
| [33] |
SHIGEMOTO I, KAWAKAMI T, OKUMURA M. A quantum chemical study on polymerization catalysts for polyesters: Catalytic performance of chelated complexes of titanium[J]. Polymer, 2013, 54(13): 3297-3305. DOI:10.1016/j.polymer.2013.04.040 |
| [34] |
GAO B, LI X, DUAN R L, et al. Titanium complexes with octahedral geometry chelated by salen ligands adopting β-cis configuration for the ring-opening polymerisation of lactide[J]. New Journal of Chemistry, 2015, 39(4): 2404-2408. DOI:10.1039/C4NJ02266A |
| [35] |
SANTORO O, REDSHAW C. Use of titanium complexes bearing diphenolate or calix [n] arene ligands in α-olefin polymerization and the ROP of cyclic esters[J]. Catalysts, 2020, 10(2): 210. DOI:10.3390/catal10020210 |
| [36] |
UPITAK K, WATTANATHANA W, NANOK T, et al. Titanium complexes of pyrrolylaldiminate ligands and their exploitation for the ring-opening polymerization of cyclic esters[J]. Dalton Transactions, 2021, 50(31): 10964-10981. DOI:10.1039/D1DT01470F |
| [37] |
MAMKHEGOV R M, MURZAKANOVA M M, TSUROVA A T, et al. Investigation of the influence of the catalyst on the kinetics of the synthesis of polyesters PET and PBT[J]. Materials Science Forum, 2018, 935: 127-133. DOI:10.4028/www.scientific.net/MSF.935.127 |
| [38] |
LI X G, SONG G, HUANG M R, et al. Cleaner synthesis and systematical characterization of sustainable poly (isosorbide-co-ethylene terephthalate) by environ-benign and highly active catalysts[J]. Journal of Cleaner Production, 2019, 206: 483-497. DOI:10.1016/j.jclepro.2018.09.046 |
| [39] |
LI X G, SONG G, HUANG M R. Cost-effective sustainable synthesis of high-performance high-molecular-weight poly (trimethylene terephthalate) by eco-friendly and highly active Ti/Mg catalysts[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(3): 2181-2195. |
| [40] |
THIELE U. Quo vadis polyester catalyst?[J]. Chemical Fibers International, 2004, 54(3): 162-163. |
| [41] |
FINELLI L, LORENZETTI C, MESSORI M, et al. Comparison between titanium tetrabutoxide and a new commercial titanium dioxide based catalyst used for the synthesis of poly (ethylene terephthalate)[J]. Journal of Applied Polymer Science, 2004, 92(3): 1887-1892. DOI:10.1002/app.20171 |
| [42] |
THAKUR A, HAMAMOTO T, IKEDA T, et al. Microwave-assisted polycondensation for screening of organically-modified TiO2/SiO2 catalysts[J]. Applied Catalysis A: General, 2020, 595: 117508. DOI:10.1016/j.apcata.2020.117508 |
| [43] |
STȨPIEŃ K, MILES C, MCCLAIN A, et al. Biocopolyesters of poly (butylene succinate) containing long-chain biobased glycol synthesized with heterogeneous titanium dioxide catalyst[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(12): 10623-10632. |
| [44] |
MOON S I, LEE C W, MIYAMOTO M, et al. Melt polycondensation of L‐lactic acid with Sn (Ⅱ) catalysts activated by various proton acids: A direct manufacturing route to high molecular weight Poly (L‐lactic acid)[J]. Journal of Polymer Science Part A: Polymer Chemistry, 2000, 38(9): 1673-1679. DOI:10.1002/(SICI)1099-0518(20000501)38:9<1673::AID-POLA33>3.0.CO;2-T |
| [45] |
MOON S I, KIMURA Y. Melt polycondensation of L-lactic acid with Sn (Ⅱ) catalysts activated by various bronsted acids [Ⅲ][J]. Journal of the Korean Polymer Society, 1999, 24(2): 78. |
| [46] |
MAHARANA T, MOHANTY B, NEGI Y S. Melt–solid polycondensation of lactic acid and its biodegradability[J]. Progress in Polymer Science, 2009, 34(1): 99-124. DOI:10.1016/j.progpolymsci.2008.10.001 |
| [47] |
KOWALSKI A, LIBISZOWSKI J, DUDA A, et al. Polymerization of L, L-dilactide initiated by tin (Ⅱ) butoxide[J]. Macromolecules, 2000, 33(6): 1964-1971. DOI:10.1021/ma991751s |
| [48] |
HYON S H, JAMSHIDI K, IKADA Y. Synthesis of polylactides with different molecular weights[J]. Biomaterials, 1997, 18(22): 1503-1508. DOI:10.1016/S0142-9612(97)00076-8 |
| [49] |
LI M M, ZHOU L, ZHANG Z Q, et al. One-step synthesis of poly (methacrylate)-b-polyester via "one organocatalyst, two polymerizations"[J]. Polymer Chemistry, 2021, 12(35): 5069-5076. DOI:10.1039/D1PY00892G |
| [50] |
GARCÍA-GALLEGO S, STENSTRÖM P, MESA-ANTUNERZ P, et al. Synthesis of heterofunctional polyester dendrimers with internal and external functionalities as versatile multipurpose platforms[J]. Biomacromolecules, 2020, 21(10): 4273-4279. DOI:10.1021/acs.biomac.0c01068 |
| [51] |
PLAJER A J, WILLIAMS C K. Heterotrimetallic carbon dioxide copolymerization and switchable catalysts: Sodium is the key to high activity and unusual selectivity[J]. Angewandte Chemie International Edition, 2021, 60(24): 13372-13379. DOI:10.1002/anie.202101180 |
| [52] |
VERT M, SCHWACH G, ENGEL R, et al. Something new in the field of PLA/GA bioresorbable polymers?[J]. Journal of Controlled Release, 1998, 53(1/2/3): 85-92. |
| [53] |
DUBEY S P, THAKUR V K, KRISHNASWAMY S, et al. Progress in environmental-friendly polymer nanocomposite material from PLA: Synthesis, processing and applications[J]. Vacuum, 2017, 146: 655-663. DOI:10.1016/j.vacuum.2017.07.009 |
| [54] |
SAMANTARAY P K, LITTLE A, HADDLETON D M, et al. Poly(glycolic acid) (PGA): A versatile building block expanding high performance and sustainable bioplastic applications[J]. Green Chemistry, 2020, 22(13): 4055-4081. DOI:10.1039/D0GC01394C |
| [55] |
YU C T, BAO J N, XIE Q, et al. Crystallization behavior and crystalline structural changes of poly (glycolic acid) investigated via temperature-variable WAXD and FTIR analysis[J]. CrystEngComm, 2016, 18(40): 7894-7902. DOI:10.1039/C6CE01623E |
| [56] |
WU D, LV Y, GUO R, et al. Kinetics of Sn (Oct)2-catalyzed ring opening polymerization of ε-caprolactone[J]. Macromolecular Research, 2017, 25(11): 1070-1075. DOI:10.1007/s13233-017-5148-z |
| [57] |
GAUTIER E, FUERTES P, CASSAGNAU P, et al. Synthesis and rheology of biodegradable poly (glycolic acid) prepared by melt ring-opening polymerization of glycolide[J]. Journal of Polymer Science Part A: Polymer Chemistry, 2009, 47(5): 1440-1449. DOI:10.1002/pola.23253 |
| [58] |
LEE C W, MASUTANI K, KIMURA Y. Ring-opening polymerization of a macrocyclic lactone monomer isolated from oligomeric byproducts of poly (butylene succinate)(PBS): An efficient route to high-molecular-weight PBS and block copolymers of PBS[J]. Polymer, 2014, 55(22): 5673-5679. DOI:10.1016/j.polymer.2014.08.028 |
| [59] |
MORALES-HUERTA J C, DE ILARDUYA A M, MUÑOZ-GUERRA S. Poly (alkylene 2, 5-furandicarboxylate) s (PEF and PBF) by ring opening polymerization[J]. Polymer, 2016, 87: 148-158. DOI:10.1016/j.polymer.2016.02.003 |
| [60] |
DE JONG E, DAM M A, SIPOS L, et al. Furandicarboxylic acid (FDCA), a versatile building block for a very interesting class of polyesters[M]. Washington: American Chemical Society, 2012: 1-13.
|
| [61] |
LEE C W, AKASHI M, KIMURA Y, et al. Synthesis and enzymatic degradability of an aliphatic/aromatic block copolyester: poly (butylene succinate)-multi-poly (butylene terephthalate)[J]. Macromolecular Research, 2017, 25(1): 54-62. DOI:10.1007/s13233-017-5011-2 |
| [62] |
VELMATHI S, NAGAHATA R, TAKEUCHI K. Extremely rapid synthesis of aliphatic polyesters by direct polycondensation of 1: 1 mixtures of dicarboxylic acids and diols using microwaves[J]. Polymer Journal, 2007, 39(8): 841-844. DOI:10.1295/polymj.PJ2006248 |
| [63] |
WANG Y J, WENG F Y, LI J X, et al. Influence of phase separation on performance of graft acrylic pressure-sensitive adhesives with various copolyester side chains[J]. ACS Omega, 2018, 3(6): 6945-6954. DOI:10.1021/acsomega.8b00737 |
| [64] |
GU C, HAUGE D A, SEVERTSON S J, et al. Effect of poly (L-lactide-co-ε-caprolactone) macromonomer composition on the properties of hot-melt adhesives with high biomass contents[J]. Industrial & Engineering Chemistry Research, 2014, 53(44): 17376-17385. |
| [65] |
PU G, HAUGE D A, GU C, et al. Influence of acrylated lactide-caprolactone macromonomers on the performance of high biomass content pressure-sensitive adhesives[J]. Macromolecular Reaction Engineering, 2013, 7(10): 515-526. DOI:10.1002/mren.201300160 |
| [66] |
WENG F Y, LI X H, WANG Y J, et al. Kinetics and modeling of ring-opening copolymerization of L-lactide and ε-caprolactone[J]. Macromolecular Reaction Engineering, 2015, 9(6): 535-544. DOI:10.1002/mren.201500009 |
| [67] |
MÖLLER M, KÅNGE R, HEDRICK J L. Sn-(OTf)2 and Sc-(OTf)3: Efficient and versatile catalysts for the controlled polymerization of lactones[J]. Journal of Polymer Science Part A: Polymer Chemistry, 2000, 38(11): 2067-2074. DOI:10.1002/(SICI)1099-0518(20000601)38:11<2067::AID-POLA150>3.0.CO;2-1 |
| [68] |
MÖLLER M, NEDERBERG F, LIM L S, et al. Stannous-(Ⅱ) trifluoromethane sulfonate: a versatile catalyst for the controlled ring-opening polymerization of lactides: Formation of stereoregular surfaces from polylactide "brushes"[J]. Journal of Polymer Science Part A: Polymer Chemistry, 2001, 39(20): 3529-3538. DOI:10.1002/pola.10003 |
| [69] |
GU Y D, NEDERBERG F, KÅNGE R, et al. Anchoring of liquid crystals on surface-initiated polymeric brushes[J]. ChemPhysChem, 2002, 3(5): 448-451. DOI:10.1002/1439-7641(20020517)3:5<448::AID-CPHC448>3.0.CO;2-0 |
| [70] |
KRICHELDORF H R, WEIDNER S M, SCHELIGA F. Synthesis of cyclic polymers and flaws of the Jacobson–Stockmayer theory[J]. Polymer Chemistry, 2020, 11(14): 2595-2604. DOI:10.1039/D0PY00226G |
| [71] |
KRICHELDORF H R, WEIDNER S M. The ring-opening polymerization–polycondensation (ROPPOC) approach to cyclic polymers[J]. Macromolecular Rapid Communications, 2020, 41(14): 2000152. DOI:10.1002/marc.202000152 |
| [72] |
KRICHELDORF H R, WEIDNER S M, SCHELIGA F. Cyclic poly (lactide) s via the ROPPOC method catalyzed by alkyl- or aryltin chlorides[J]. Journal of Polymer Science Part A: Polymer Chemistry, 2019, 57(9): 952-960. DOI:10.1002/pola.29347 |
| [73] |
KRICHELDORF H R, WEIDNER S M. Cyclic poly(L-lactide)s via simultaneous ROP and polycondensation (ROPPOC) catalyzed by dibutyltin phenoxides[J]. European Polymer Journal, 2018, 109: 360-366. DOI:10.1016/j.eurpolymj.2018.10.005 |
| [74] |
KRICHELDORF H R, WEIDNER S M. Syntheses of polylactides by means of tin catalysts[J]. Polymer Chemistry, 2022, 13(12): 1618-1647. DOI:10.1039/D2PY00092J |
| [75] |
LAI S Q, GAO Y, YUE L. Heterogeneous catalytic synthesis of poly (butylene succinate) by attapulgite‐supported Sn catalyst[J]. Journal of Applied Polymer Science, 2015, 132(13): 41729. DOI:10.1002/app.41729 |
| [76] |
杨英, 李桂顺, 佘长坤, 等. 高品质聚酯生产用纳米GeO2催化材料的制备及挑战[J]. 材料科学, 2021, 11(3): 209-218. YANG Y, LI G S, SHE C K, et al. Synthesis and challenges of nano-GeO2 catalysts used for producing high-quality polyesters[J]. Material Sciences, 2021, 11(3): 209-218. |
| [77] |
ARKLES B, LARSON G. Metal-organics for materials, polymers & synthesis[M]. Morrisville: Gelest, Inc, 2016: 51.
|
| [78] |
JACQUEL N, FREYERMOUTH F, FENOUILLOT F, et al. Synthesis and properties of poly (butylene succinate): Efficiency of different transesterification catalysts[J]. Journal of Polymer Science Part A: Polymer Chemistry, 2011, 49(24): 5301-5312. DOI:10.1002/pola.25009 |
| [79] |
vKRICHELDORF H R, LANGANKE D. Polylactones 54: ring-opening and ring-expansion polymerizations of ε-caprolactone initiated by germanium alkoxides[J]. Polymer, 2002, 43(6): 1973-1977. DOI:10.1016/S0032-3861(01)00745-5 |
| [80] |
SHIGEMOTO I, KAWAKAMI T, TAIKO H, et al. A quantum chemical study on the polycondensation reaction of polyesters: The mechanism of catalysis in the polycondensation reaction[J]. Polymer, 2011, 52(15): 3443-3450. DOI:10.1016/j.polymer.2011.05.055 |
| [81] |
NISHIWAKI Y, KIMURA Y, MASUTANI K, et al. High‐molecular‐weight poly (1, 2‐propylene succinate): A soft biobased polyester applicable as an effective modifier of poly (L‐lactide)[J]. Journal of Polymer Science Part A: Polymer Chemistry, 2018, 56(16): 1795-1805. DOI:10.1002/pola.29060 |
| [82] |
GUO J, HAQUETTE P, MARTIN J, et al. Replacing tin in lactide polymerization: Design of highly active germanium‐based catalysts[J]. Angewandte Chemie International Edition, 2013, 52(51): 13584-13587. DOI:10.1002/anie.201306623 |
| [83] |
FINNE A, REEMA, ALBERTSSON A C. Use of germanium initiators in ring‐opening polymerization of L‐lactide[J]. Journal of Polymer Science Part A: Polymer Chemistry, 2003, 41(19): 3074-3082. DOI:10.1002/pola.10887 |
| [84] |
WANG J, WANG Q Y, WANG G Y. MIL-53(Al) catalytic synthesis of poly (ethylene terephthalate)[J]. Turkish Journal of Chemistry, 2022, 46(4): 1281-1290. DOI:10.55730/1300-0527.3434 |
| [85] |
XIAO B, WANG L P, MEI R H, et al. Ethylene glycol aluminum as a novel catalyst for the synthesis of poly (ethylene terephthalate)[J]. Chinese Chemical Letters, 2011, 22(6): 741-744. DOI:10.1016/j.cclet.2010.12.036 |
| [86] |
CHEN X L, WANG B, PAN L, et al. Synthesis of unsaturated (co) polyesters from ring-opening copolymerization by aluminum bipyridine bisphenolate complexes with improved protonic impurities tolerance[J]. Macromolecules, 2022, 55(9): 3502-3512. DOI:10.1021/acs.macromol.2c00034 |
| [87] |
ANDREA K A, PLOMMER H, KERTON F M. Ring-opening polymerizations and copolymerizations of epoxides using aluminum-and boron-centered catalysts[J]. European Polymer Journal, 2019, 120: 109202. DOI:10.1016/j.eurpolymj.2019.08.029 |
| [88] |
HADOR R, BOTTA A, VENDITTO V, et al. The Dual-Stereocontrol mechanism: Heteroselective polymerization of rac-Lactide and syndioselective polymerization of meso-lactide by chiral aluminum salan catalysts[J]. Angewandte Chemie International Edition, 2019, 131(41): 14821-14827. |
| [89] |
LIN Q H, GU Y Q, CHEN D J. Attapulgite-supported aluminum oxide hydroxide catalyst for synthesis of poly (ethylene terephthalate)[J]. Journal of Applied Polymer Science, 2013, 129(5): 2571-2579. DOI:10.1002/app.38973 |
| [90] |
NEDERBERG F, CONNOR E F, MÖLLER M, et al. New paradigms for organic catalysts: The first organocatalytic living polymerization[J]. Angewandte Chemie International Edition, 2001, 40(14): 2712-2715. DOI:10.1002/1521-3773(20010716)40:14<2712::AID-ANIE2712>3.0.CO;2-Z |
| [91] |
KIESEWETTER M, KIESEWETTER E, FASTNACHT K, et al. Convergence of high rate and selectivity in organocatalytic ring-opening polymerization: Abstracts of Papers of the American Chemical Society [C]. Washington: American Chemical Society, 2017: 253.
|
| [92] |
KIESEWETTER M K, SHIN E J, HEDRICK J L, et al. Organocatalysis: Opportunities and challenges for polymer synthesis[J]. Macromolecules, 2010, 43(5): 2093-2107. DOI:10.1021/ma9025948 |
| [93] |
LOHMEIJER B G G, PRATT R C, LEIBFARTH F, et al. Guanidine and amidine organocatalysts for ring-opening polymerization of cyclic esters[J]. Macromolecules, 2006, 39(25): 8574-8583. DOI:10.1021/ma0619381 |
| [94] |
DZIENIA A, MAKSYM P, HACHUŁA B, et al. Studying the catalytic activity of DBU and TBD upon water-initiated ROP of ε-caprolactone under different thermodynamic conditions[J]. Polymer Chemistry, 2019, 10(44): 6047-6061. DOI:10.1039/C9PY01134J |
| [95] |
DOVE A P, LI H B, PRATT R C, et al. Stereoselective polymerization of rac- and meso-lactide catalyzed by sterically encumbered N-heterocyclic carbenes[J]. Chemical Communications, 2006(27): 2881-2883. DOI:10.1039/b601393g |
| [96] |
REN C L, ZHU X, ZHAO N, et al. Polystyrene beads supported phosphazene superbase as recyclable organocatalyst for ring-opening polymerization of δ-valerolactone[J]. European Polymer Journal, 2019, 119: 130-135. DOI:10.1016/j.eurpolymj.2019.07.022 |
| [97] |
SUSPERREGUI N, DELCROIX D, MARTÍN-VACA B, et al. Ring-opening polymerization of ε-caprolactone catalyzed by sulfonic acids: Computational evidence for bifunctional activation[J]. The Journal of Organic Chemistry, 2010, 75(19): 6581-6587. DOI:10.1021/jo101346t |
| [98] |
GAZEAU-BUREAU S, DELCROIX D, MARTÍN-VACA B, et al. Organo-catalyzed ROP of ε-caprolactone: Methanesulfonic acid competes with trifluoromethanesulfonic acid[J]. Macromolecules, 2008, 41(11): 3782-3784. DOI:10.1021/ma800626q |
| [99] |
MAKIGUCHI K, SATOH T, KAKUCHI T. Diphenyl phosphate as an efficient cationic organocatalyst for controlled/living ring-opening polymerization of δ-valerolactone and ε-caprolactone[J]. Macromolecules, 2011, 44(7): 1999-2005. DOI:10.1021/ma200043x |
| [100] |
DELCROIX D, COUFFIN A, SUSPERREGUI N, et al. Phosphoric and phosphoramidic acids as bifunctional catalysts for the ring-opening polymerization of ε-caprolactone: a combined experimental and theoretical study[J]. Polymer Chemistry, 2011, 2(10): 2249-2256. DOI:10.1039/c1py00210d |
| [101] |
BOURISSOU D, MARTIN-VACA B, DUMITRESCU A, et al. Controlled cationic polymerization of lactide[J]. Macromolecules, 2005, 38(24): 9993-9998. DOI:10.1021/ma051646k |
| [102] |
ZHANG J, XU L G, XIAO W H, et al. Ring-opening polymerization of ε-caprolactone with recyclable and reusable squaric acid organocatalyst[J]. European Polymer Journal, 2021, 157: 110643. DOI:10.1016/j.eurpolymj.2021.110643 |
| [103] |
MEZZASALMA L, HARRISSON S, SABA S, et al. Bulk organocatalytic synthetic access to statistical copolyesters from L-lactide and ε-caprolactone using benzoic acid[J]. Biomacromolecules, 2019, 20(5): 1965-1974. DOI:10.1021/acs.biomac.9b00190 |
| [104] |
WANG Y J, XIA M, KONG X Q, et al. Tailoring chain structures of L-lactide and ε-caprolactone copolyester macromonomers using rac-binaphthyl-diyl hydrogen phosphate-catalyzed ring-opening copolymerization with monomer addition strategy[J]. RSC Advances, 2017, 7(46): 28661-28669. DOI:10.1039/C7RA05531E |
| [105] |
王欢, 吴云雁, 赵燕飞, 等. 离子液体介导CO2化学转化研究进展[J]. 物理化学学报, 2021, 37(5): 2010022. WANG H, WU Y Y, ZHAO Y F, et al. Recent progress on ionic liquid-mediated CO2 conversion[J]. Acta Physico-Chimica Sinica, 2021, 37(5): 2010022. |
| [106] |
QU X L, JIANG M, WANG B, et al. A Brønsted acidic ionic liquid as an efficient and selective catalyst system for bioderived high molecular weight poly(ethylene 2, 5-furandicarboxylate)[J]. ChemSusChem, 2019, 12(22): 4927-4935. DOI:10.1002/cssc.201902020 |
| [107] |
SONG P F, CHEN Y L, LI Y L, et al. A one-pot strategy to synthesize block copolyesters from monomer mixtures using a hydroxy-functionized ionic liquid[J]. Macromolecular Rapid Communications, 2020, 41(23): 2000436. DOI:10.1002/marc.202000436 |
| [108] |
WEI C Y, LIU Z P, TAN H W, et al. A non-metal route to realize the bio-based polyester of poly(hexylene succinate): Preparation conditions, side-reactions and mechanism in sulfonic acid-functionalized Brønsted acidic ionic liquids[J]. RSC Advances, 2020, 10(58): 35381-35388. DOI:10.1039/D0RA07157A |
| [109] |
HIROSHI U, SHIRO K. Enzymatic ring-opening polymerization of lactones catalyzed by lipase[J]. Chemistry Letters, 1993, 22(7): 1149-1150. DOI:10.1246/cl.1993.1149 |
| [110] |
KNANI D, KOHN D H. Enzymatic polyesterification in organic media. Ⅱ. Enzyme-catalyzed synthesis of lateral-substituted aliphatic polyesters and copolyesters[J]. Journal of Polymer Science Part A: Polymer Chemistry, 1993, 31(12): 2887-2897. DOI:10.1002/pola.1993.080311202 |
| [111] |
GROSS R A, GANESH M, LU W H. Enzyme-catalysis breathes new life into polyester condensation polymerizations[J]. Trends in Biotechnology, 2010, 28(8): 435-443. DOI:10.1016/j.tibtech.2010.05.004 |
| [112] |
COMERFORD J W, BYRNE F P, WEINBERGER S, et al. Thermal upgrade of enzymatically synthesized aliphatic and aromatic oligoesters[J]. Materials, 2020, 13(2): 368. DOI:10.3390/ma13020368 |
| [113] |
HEVILLA V, SONSECA A, ECHEVERRÍA C, et al. Enzymatic synthesis of polyesters and their bioapplications: Recent advances and perspectives[J]. Macromolecular Bioscience, 2021, 21(10): 2100156. DOI:10.1002/mabi.202100156 |
| [114] |
SCHMIDT S, SCHERKUS C, MUSCHIOL J, et al. An enzyme cascade synthesis of ε-caprolactone and its oligomers[J]. Angewandte Chemie International Edition, 2015, 54(9): 2784-2787. DOI:10.1002/anie.201410633 |
| [115] |
SCHMIDT S, BÜCHSENSCHÜTZ H C, SCHERKUS C, et al. Biocatalytic access to chiral polyesters by an artificial enzyme cascade synthesis[J]. ChemCatChem, 2015, 7(23): 3951-3955. DOI:10.1002/cctc.201500823 |
| [116] |
ZHONG Y L, FENG Q Y, WANG X Q, et al. Functionalized polyesters via stereoselective electrochemical ring-opening polymerization of o-carboxyanhydrides[J]. ACS Macro Letters, 2020, 9(8): 1114-1118. DOI:10.1021/acsmacrolett.0c00364 |
| [117] |
QI M, DONG Q, WANG D W, et al. Electrochemically switchable ring-opening polymerization of lactide and cyclohexene oxide[J]. Journal of the American Chemical Society, 2018, 140(17): 5686-5690. DOI:10.1021/jacs.8b02171 |
| [118] |
NOËL T, ZYSMAN-COLMAN E. The promise and pitfalls of photocatalysis for organic synthesis[J]. Chem Catalysis, 2022, 2(3): 468-476. DOI:10.1016/j.checat.2021.12.015 |
| [119] |
ZHANG Y C, BRADLEY M, GENG J. Photo-controlled one-pot strategy for the synthesis of asymmetric three-arm star polymers[J]. Polymer Chemistry, 2019, 10(35): 4769-4773. DOI:10.1039/C9PY00774A |
| [120] |
SUNITHA K, NAIR C P R. Synthetic applications of click chemistry in thermosetting block and graft polymers[M]. Oxford: William Andrew, 2022: 931-952.
|
| [121] |
BAYSAK E, DURMAZ H, TUNCA U, et al. Synthesis of activated ester functional polyesters through light-induced 4+4 cycloaddition polymerization[J]. Macromolecular Chemistry and Physics, 2017, 218(18): 1600572. DOI:10.1002/macp.201600572 |
| [122] |
KRICHELDORF H R. Ring-opening polycondensations[J]. Macromolecular Rapid Communications, 2000, 21(9): 528-541. DOI:10.1002/1521-3927(20000601)21:9<528::AID-MARC528>3.0.CO;2-S |
| [123] |
SHIN E J, JEONG W, BROWN H A, et al. Crystallization of cyclic polymers: Synthesis and crystallization behavior of high molecular weight cyclic poly (ε-caprolactone) s[J]. Macromolecules, 2011, 44(8): 2773-2779. DOI:10.1021/ma102970m |
| [124] |
CASTRO-OSMA J A, ALONSO-MORENO C, GARCÍA-MARTINEZ J C, et al. Ring-opening (ROP) versus ring-expansion (REP) polymerization of ε-caprolactone to give linear or cyclic polycaprolactones[J]. Macromolecules, 2013, 46(16): 6388-6394. DOI:10.1021/ma401216u |
| [125] |
MISAKA H, KAKUCHI R, ZHANG C H, et al. Synthesis of well-defined macrocyclic poly (δ-valerolactone) by "click cyclization"[J]. Macromolecules, 2009, 42(14): 5091-5096. DOI:10.1021/ma900712p |
| [126] |
WANG H D, ZHANG L, LIU B Y, et al. Synthesis of high molecular weight cyclic poly(ε-caprolactone)s of variable ring size based on a light-induced ring-closure approach[J]. Macromolecular Rapid Communications, 2015, 36(18): 1646-1650. DOI:10.1002/marc.201500171 |
| [127] |
JOSSE T, ALTINTAS O, OEHLENSCHLAEGER K K, et al. Ambient temperature catalyst-free light-induced preparation of macrocyclic aliphatic polyesters[J]. Chemical Communications, 2014, 50(16): 2024-2026. DOI:10.1039/c3cc49067j |
| [128] |
METAXAS A C, MEREDITH R J. Industrial microwave heating[M]. London: IET, 1983.
|
| [129] |
NAKAMURA T, NAGAHATA R, TAKEUCHI K. Microwave-assisted polyester and polyamide synthesis[J]. Mini-Reviews in Organic Chemistry, 2011, 8(3): 306-314. DOI:10.2174/157019311796197454 |
| [130] |
WIESBROCK F, HOOGENBOOM R, SCHUBERT U S. Microwave-assisted polymer synthesis: state-of-the-art and future perspectives[J]. Macromolecular Rapid Communications, 2004, 25(20): 1739-1764. DOI:10.1002/marc.200400313 |
| [131] |
KÉKI S, BODNÁR I, BORDA J, et al. Fast microwave-mediated bulk polycondensation of D, L-lactic acid[J]. Macromolecular Rapid Communications, 2001, 22(13): 1063-1065. DOI:10.1002/1521-3927(20010901)22:13<1063::AID-MARC1063>3.0.CO;2-3 |
| [132] |
YAMADA S, TAKASU A, TAKAYAMA S, et al. Microwave-assisted solution polycondensation of l-lactic acid using a Dean–Stark apparatus for a non-thermal microwave polymerization effect induced by the electric field[J]. Polymer Chemistry, 2014, 5(18): 5283-5288. DOI:10.1039/C4PY00639A |
| [133] |
ESPINOSA-LÓPEZ A C, ÁVILA-ORTA C A, MEDELLÍN-RODRÍGUEZ F J, et al. Microwave-assisted esterification step of poly(ethylene terephthalate) (PET) synthesis through ethylene glycol and terephthalic acid[J]. Polymer Bulletin, 2019, 76(6): 2931-2944. DOI:10.1007/s00289-018-2521-9 |
| [134] |
NAGAHATA R, NAKAMURA T, TAKEUCHI K. Microwave-assisted rapid synthesis of poly(butylene succinate): principal effect of microwave irradiation of accelerating the polycondensation reaction[J]. Polymer Journal, 2018, 50(5): 347-354. DOI:10.1038/s41428-018-0024-z |
| [135] |
王松林, 吴海强, 姜冰雪, 等. 聚酯链结构定制及其构效关系[J]. 化工学报, 2021, 72(2): 852-862. WANG S L, WU H Q, JIANG B X, et al. Tailoring chain structures of polyesters and their effect on physical and degradation properties[J]. CIESC Journal, 2021, 72(2): 852-862. |