2. 陕西北元化工集团股份有限公司, 陕西 榆林 719319
2. Shaanxi Beiyuan Chemical Group Limited, Yulin 719319, China
海水淡化、煤化工、工业冷却和油田开采过程中会产生大量浓盐水,其中有些含有挥发性有机物和重金属等污染物。渗透汽化(pervaporation,PV)可浓缩处理含盐有机废水并减量化,同时得到纯水。渗透汽化主要优势在于,对于一价盐截留率通常超过99%,与反渗透相比,渗透汽化过程不需要克服盐水的渗透压,因此净水回收率高。与使用疏水多孔膜的膜蒸馏相比,渗透汽化采用亲水性致密膜,在截留挥发性有机物和抗污染性方面具有优势[1],避免了膜蒸馏在长时间运行时可能产生的润湿和孔阻塞现象。通过构建合适的膜结构,可以提高膜材料对水分子的优先透过能力,从而获得较高的选择性和产水纯度[1-5]。渗透汽化可以利用低品位热源,如地热能、太阳能、工业废热等,从而大幅节约能耗,使能耗与反渗透(reverse osmosis,RO)等过程相当[6]。渗透汽化脱盐膜包括有机、无机和杂化材料[1]。近些年氧化石墨烯(graphene oxide,GO)膜在水处理方面显示了出色的分离性能[7-8]。GO片层表面氧化区上的官能团起到捕捉水分子的能力,使层间距增大,有利于水分子在层间插入[9-10]。未氧化区具有疏水性,作用相当于毛细管网,使得水分子能够在其中近乎自由扩散[11-12],因此可实现水分子快速传输。由于层状GO膜在水中发生溶胀,导致层层堆叠结构被破坏[13-14]。利用共价键[2-3, 5, 15-23]、金属离子配位作用[13, 24-26]和石墨片层间的π-π堆叠作用[14, 27-29]等插层交联方法,可以提高GO膜的耐溶胀性和机械稳定性。目前,将层状GO膜用于处理实际废水的研究主要集中于纳滤过程[30-31],在渗透汽化方面研究较少。为此,本文用压力辅助自组装法制备了层状GO膜,以亲水的柔性聚乙烯醇(poly(vinyl alcohol),PVA)为插层分子,增大GO膜的片层间距,并且提供大量交联位点。以戊二醛(glutaraldehyde,GA)为交联剂,与GO和PVA上的羟基反应生成缩醛。基于该柔性大分子插层和交联改性策略,提高了GO膜的水通量和结构稳定性。考察层状GO膜对各种废水的处理能力,结果表明该膜在渗透汽化脱盐处理浓盐水和实际废水方面具有良好应用前景。
2 实验部分 2.1 实验原料实验所用材料和试剂有石墨(分析纯),天津化学试剂一厂;高锰酸钾(分析纯)、硫酸(~98%,分析纯)、硝酸钠(分析纯)、盐酸(~36%,分析纯),西陇化工有限公司;过氧化氢(30%,分析纯)、聚乙烯醇(PVA,聚合度1 700,醇解度97%)、戊二醛(GA,25%水溶液),国药集团化学试剂有限公司。
2.2 GO的制备在烧杯中加入6 g石墨粉,再加入3 g硝酸钠和138 mL浓硫酸,在冰浴环境下搅拌1 h,其间分批次缓慢加入18 g高锰酸钾,继续搅拌2 h。升温至35 ℃并搅拌3 h,然后向反应液中缓慢加入276 mL去离子水,控制反应液温度不超过70 ℃。再升温至95 ℃搅拌30 min后,用840 mL去离子水稀释反应液,然后加入60 mL质量分数为30%的过氧化氢溶液。将得到的悬浮液静置沉降,倾析出上层清液。对沉淀物用质量分数为3%的盐酸洗涤沉降3次,用去离子水洗涤沉降至上层液为中性。将下层沉淀在转速为10 000 r·min-1下离心,沉淀物在50 ℃真空干燥,用球磨机研磨,得到棕色GO粉末。
2.3 层状GO-PVA膜的制备将GO粉末加水超声4 h,配制成质量浓度为0.5 g·L-1的水分散液。将PVA固体加水在90 ℃下搅拌4 h,制得质量浓度为0.5 g·L-1 PVA水溶液。基于Sun等[32]的前期工作,选择铸膜液中GO与PVA质量比为2:10,即向200 mL去离子水中加入2 mL GO水分散液和10 mL PVA溶液摇匀。以标称孔径0.22 μm的混合纤维素微滤膜为基膜,将上述溶液在0.1 MPa下氮气瓶加压过滤通过基膜,取出膜后在50 ℃干燥,制得GO-PVA膜。同样条件下,不加入PVA,制备纯GO膜进行对比。在交联步骤中,将预浸泡好的膜放入200 mL去离子水、800 μL GA溶液和2 mL盐酸的混合溶液中,50 ℃下反应4 h。GA在酸性条件下与GO和PVA上的羟基反应,生成缩醛(如图 1所示)。用去离子水冲洗膜,50 ℃下烘干得到交联GO-GA膜和GO-PVA-GA膜。
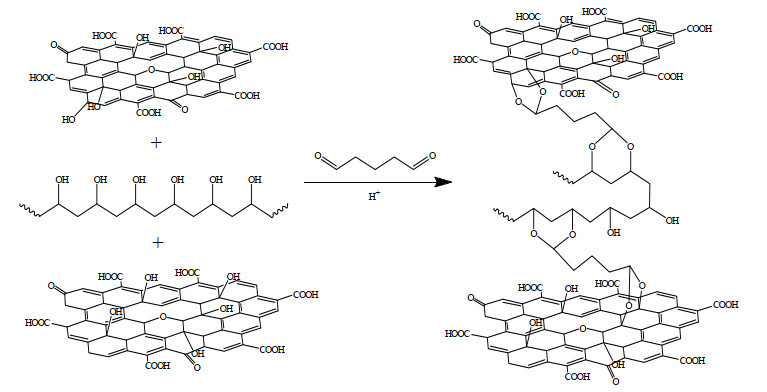
|
图 1 PVA插层GO膜的交联反应机理 Fig.1 Cross-linking mechanism of PVA intercalated with GO membrane |
采用场发射扫描电子显微镜(TESCAN MAIA3LMH)分析膜表面形貌。利用全反射傅里叶变换红外光谱仪(Nicolet iS50)表征膜表面化学结构,扫描波数范围为4 000~650 cm-1。采用X射线衍射仪(Shimadzu XRD-6100)测试GO的层间距,采用Cu靶,X射线波长0.154 178 nm,扫描范围为6°~35°,扫描速度为10 (°)·min-1。使用光学接触角测试仪(KRÜSS DSA100)测试膜表面接触角。上述样品测试前均经50 ℃真空干燥处理。
2.5 渗透汽化脱盐实验真空式渗透汽化实验装置如图 2所示。有效膜面积为19.64 cm2。通过蠕动泵使原料液以16 L·h-1的流率进入膜池循环流动,透过侧通过真空泵保持0.095 MPa真空度,运行30 min后开始记录数据。
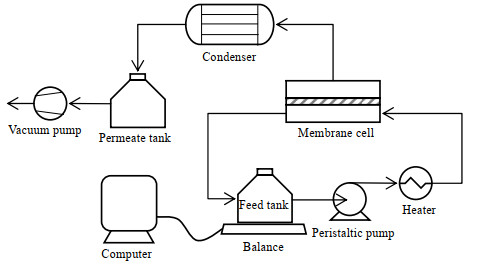
|
图 2 渗透汽化实验装置示意图 Fig.2 Schematic diagram of the pervaporation setup |
用水通量和溶质截留率表征膜的分离性能。水通量J (kg·m-2·h-1)的计算公式如下:
| $J = \frac{m}{{A \cdot t}}$ |
式中:m为料液罐中料液质量随时间的减少量(kg),A为膜的有效面积(m2),t为测试时间(h)。
溶质截留率:
| $R = \frac{{{c_\rm f} - {c_\rm p}}}{{{c_\rm f}}} \times 100\% $ |
式中:R为截留率;cf为原料液中组分的物质的量浓度(mol·L-1),cp为透过液中组分的物质的量浓度(mol·L-1)。盐截留率测定采用电导率仪(Ohaus DDS-307);COD检测采用COD快速测定仪(连华科技5B-3F);总有机碳(TOC)检测采用总有机碳分析仪(Shimadzu TOCL-CPN);氨氮(NH3-N)检测采用紫外可见分光光度计(北京普析DSPC-TU1810);汞含量检测采用原子荧光光谱仪(北分瑞利AF-640A)。
2.6 膜稳定性和水处理性能评价将GO-PVA-GA膜放在质量分数为20% 的NaCl溶液中常温下浸泡20 d,考察浸泡后的渗透汽化脱盐性能。为考察耐酸碱性,将GO-PVA-GA膜浸泡在pH=1、7、13的盐酸、去离子水以及氢氧化钠溶液中12 h,浸泡完成后进行渗透汽化脱盐测试。渗透汽化测试连续运行15 h (65 ℃,质量分数为10%的NaCl溶液),考察膜的长期运行稳定性。另外,分别采用化工企业产生的含盐有机废水、含汞废水和RO浓排水作为原料液,考察GO膜处理实际废水的性能。
3 结果与讨论 3.1 层状GO-PVA膜的物理化学结构合成得到的GO纳米片尺寸为1~3 μm片层,具有褶皱形貌(如图 3(a)、(b)所示)。将GO纳米片分散在水中,通过压力辅助过滤,使GO片层层堆叠在基膜上,得到GO膜。SEM图像显示其表面具有独特的褶皱结构(图 3(d)),从膜的断面形貌可以看出GO在基膜上形成了超薄的层层堆叠结构,PVA插层后皮层变厚,GO膜厚度110.1 nm,插层交联后厚度增大到140.1 nm(图 3(e)、(f))。
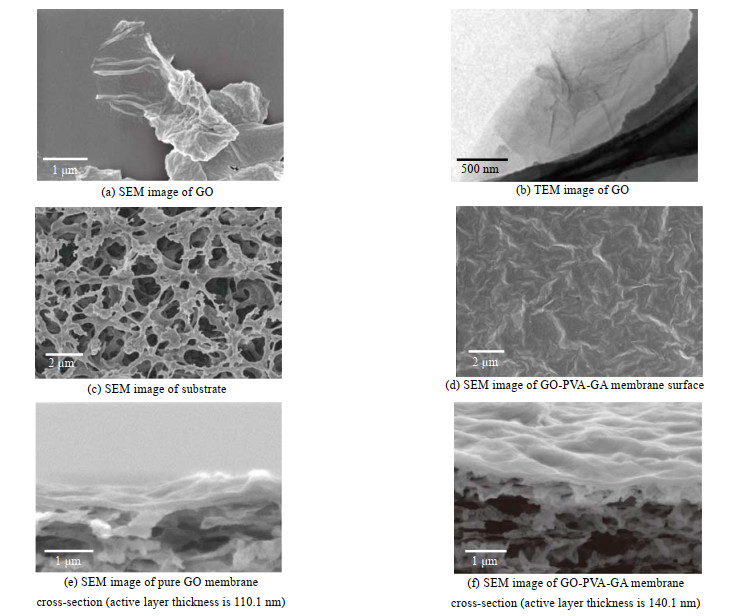
|
图 3 GO纳米片、纯GO膜和GO-PVA-GA膜的形貌 Fig.3 Images of GO sheets, GO membranes and GO-PVA-GA membranes |
如图 4所示为红外光谱特征峰显示GO膜在3 349、1 270、1 071 cm-1处分别存在羟基峰、环氧峰和烷氧峰[33-34],1 647 cm-1处存在GO未氧化区域的稠环芳烃峰[34],2 920 cm-1处存在来自GA和PVA的─CH2─特征吸收峰[32]。如图 5所示,PVA分子中富含亲水性羟基,插层后GO-PVA膜水接触角降低,经过交联的GO-GA和GO-PVA-GA膜的水接触角略微大于GO和GO-PVA膜,这是因为交联过程消耗了GO的亲水基团羟基,使亲水性减弱。
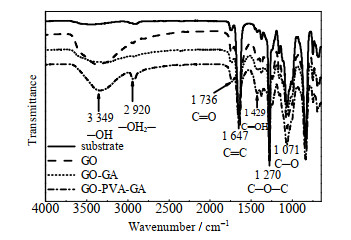
|
图 4 GO膜及基膜的红外光谱 Fig.4 FTIR spectra of GO membranes |
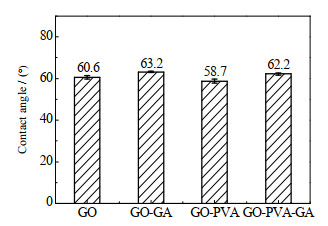
|
图 5 GO膜的水接触角 Fig.5 Water contact angles of GO membranes |
如图 6(a)、(b)所示对比了GO膜和PVA插层GO膜在交联前后的XRD图谱,由Bragg公式计算得到膜的层间距。从图 6中可看出,PVA插层使膜的层间距均不同程度增大,说明PVA分子拓宽了GO片层之间的距离,有利于提高水渗透性。GO和PVA具有丰富的含氧基团,水分子通过氢键进入层间,使膜发生溶胀,因此膜在湿态下层间距均高于干态。与干膜相比,润湿后,GO膜层间距增大55.6%,GO-GA膜层间距增大34.6%,而GO-PVA膜仅增加16.7%,说明GA交联和PVA插层均可一定幅度抑制膜的溶胀。通过对比发现,GO-PVA-GA膜的层间距增大幅度最小,仅为4.7%,这是由于PVA有丰富的羟基供GA交联,二者的协同作用大幅提高交联程度,因此可显著抑制膜的溶胀。
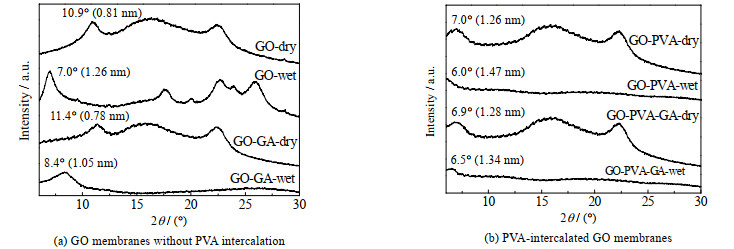
|
图 6 膜在干燥和湿润状态下的XRD图谱 Fig.6 XRD patterns of GO membranes at dry and wet states |
由XRD表征结果可知GO-PVA-GA膜具有较大层间距和较好的抗溶胀能力,这将有利于水分子传递,且具有较高的结构稳定性,因此选择该膜,考察其处理不同质量分数NaCl溶液和各种含盐废水的渗透汽化性能。如图 7所示,随着NaCl溶液质量分数的提高,GO-PVA-GA交联膜的盐截留率均达到99.99%,水通量逐渐下降,这是由于盐浓度升高使水的活度降低,水在膜两侧的化学势差减小,导致传质推动力减小。不过,当质量分数达到20% 时,通量仍可达到28.1 kg·m-2·h-1,因此在处理高浓盐水方面具有应用潜力。
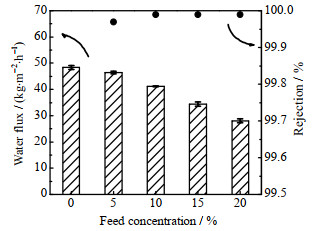
|
图 7 处理不同质量分数的NaCl溶液的渗透汽化通量与盐截留率(进料温度为65 ℃) Fig.7 Water flux and salt rejection of membranes for NaCl solution treatment via pervaporation (feed temperature is 65 ℃) |
如图 8所示,随着进料温度从60 ℃升高至85 ℃,膜的通量升高至98.2 kg·m-2·h-1,盐截留率始终保持为99.99%。渗透汽化推动力与膜两侧蒸气压差有关,料液温度升高,原料侧水的蒸气压上升,透过侧近似为零,因此传质推动力随温度快速上升。此外,温度升高使水分子和PVA链段运动加剧,水分子更容易透过膜。
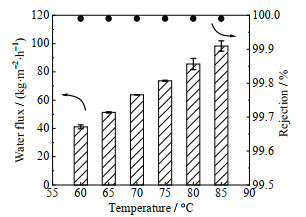
|
图 8 不同进料温度下的渗透汽化通量与盐截留率(进料NaCl质量分数为10%) Fig.8 Water flux and salt rejection of membranes for pervaporation desalination under different temperatures (NaCl concentration is 10%) |
如图 9所示,二维层状膜在高水通量方面具有显著的优势。二维层状膜的超薄皮层传质阻力较小,其层状结构形成的巨大毛细管力能促进水分子传递[35-36],表 1中对比了本研究和文献报道的层状GO膜的渗透汽化脱盐性能。通过PVA分子插层,可以增大GO层间距,并且保持较高的亲水性,因此GO-PVA-GA膜显示出较高的水通量(图 9)。
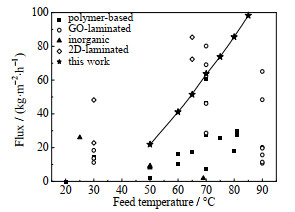
|
图 9 GO-PVA-GA膜渗透汽化脱盐通量性能与文献对比[3, 37-51] Fig.9 Pervaporation desalination of GO-PVA-GA membrane and membranes from literature |
|
|
表 1 层状GO膜渗透汽化脱盐性能对比 Table 1 Comparison of GO-laminated membrane performance for pervaporation desalination |
如表 2所示,将膜在65 ℃、pH=1的盐酸溶液中浸泡12 h后,渗透汽化通量和盐截留率保持稳定,截留率为99.99%,说明GO-PVA-GA在酸环境下具有良好的化学稳定性。但在pH=13的碱性条件下,65 ℃下膜结构被破坏,说明该复合膜耐受碱性环境能力较弱。如图 10(a)所示,GO-PVA-GA膜在经过长达20 d质量分数为20%的浓盐水浸泡后,膜的通量与截留率均没有明显变化,说明膜可以耐受长时间高浓盐水环境。图 10(b)显示,GO-PVA-GA膜在长时间处理质量分数为10% 的NaCl溶液时运行性能稳定,渗透汽化通量平均为42 kg·m-2·h-1,截留率始终保持在99.9% 以上。
|
|
表 2 GO-PVA-GA膜的耐酸碱性测试结果 Table 2 pH resistance of the GO-PVA-GA membrane (feed temperature 65 ℃) |
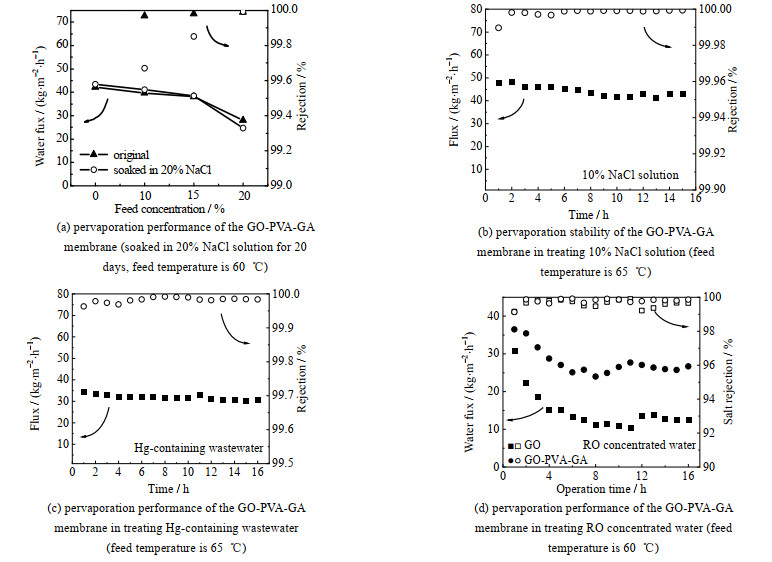
|
图 10 GO膜和GO-PVA-GA膜渗透汽化性能和长期运行稳定性 Fig.10 Pervaporation performance of the GO and GO-PVA-GA membranes |
电石法聚氯乙烯生产工艺产生的含汞废水成分复杂,由于汞单质及汞化合物具有挥发性,传统蒸发结晶技术难以深度脱除痕量汞。使用该膜处理微汞废水(汞含量3.2 μg·L-1;电导率113.9 mS·cm-1),如图 10(c)所示,在20 h的测试过程中,平均通量为30 kg·m-2·h-1,透过液电导率降至60.3 μS·cm-1,汞含量降至0.01 μg·L-1,对溶质截留率和汞脱除率分别达到99.9% 和99.7%,处理后的产水可直接回用至生产工段,实现废水零排放。
反渗透过程排放大量浓缩水需要回收利用,以提高水回用率和减少对环境的不利影响。采用GO-PVA-GA膜处理反渗透浓排水,如图 10(d)显示,与GO膜相比,GO-PVA-GA膜在经过16 h的连续运行后,通量轻微下降,最终稳定在25 kg·m-2·h-1左右,相较于GO膜的通量提高90% 以上,溶质截留率为99.8%。每间隔一定时间取样测定透过侧COD及氨氮值,结果如表 3所示,产水中的COD脱除率约80%,NH3-N脱除率约97%,透过侧水质远高于设计排放标准。在RO浓水测试之前,GO-PVA-GA膜在60 ℃渗透汽化处理质量分数为5%的NaCl溶液,水通量为39.73 kg·m-2·h-1。将长期测试的膜用去离子水冲洗膜表面,相同条件下再处理质量分数为5% 的NaCl溶液,水通量为37.1 kg·m-2·h-1,基本恢复到初始水平,说明该膜具有良好的耐污染性。
|
|
表 3 GO-PVA-GA膜处理RO浓排水时透过液的COD与NH3-N值随运行时间变化 Table 3 COD and NH3-N values of permeated water from GO-PVA-GA treated RO concentrated water |
使用GO-PVA-GA膜对2种不同浓度的有机含盐染料废水(主要含有NaCl和有机物)进行渗透汽化处理,也表现出高的水通量和杂质截留率。如表 4所示,从图中可知,经过一步法渗透汽化处理,浅黄色和红褐色的污水样品变为透明无色,COD、TOC及电导率大幅降低,证明有机含盐染料污水中的盐和有机物均得到有效截留。上述实验结果表明GO-PVA-GA膜具有优异的污水处理能力,不但能够处理高浓盐水(质量分数>10%),而且对废水中的挥发性汞和有机物也有较高的截留能力。
|
|
表 4 污水样品处理前后对比 Table 4 Comparison of sewage samples before and after pervaporation |
采用压力辅助自组装法制备了聚乙烯醇插层的氧化石墨烯层状膜,并用戊二醛对其进行交联。红外吸收光谱分析表明膜内形成了交联结构。XRD测试表明加入聚乙烯醇,使得膜的层间距增大。聚乙烯醇分子的羟基提供丰富的交联位点,在二维通道内形成稳定的三维交联结构,使层状氧化石墨烯膜的溶胀得到有效抑制。该交联膜具有良好的物化稳定性,在强酸环境和高浓盐水中浸泡后仍能保持稳定的分离性能。该膜能有效处理不同类型的工业污水,对反渗透浓排水和有机废水处理表现出良好的分离性能与耐污染性,表明该膜在处理浓盐水和污水方面具有实际应用前景。
| [1] |
WANG Q Z, LI N, BOLTO B, et al. Desalination by pervaporation: A review[J]. Desalination, 2016, 387: 46-60. DOI:10.1016/j.desal.2016.02.036 |
| [2] |
FENG B, XU K, HUANG A. Covalent synthesis of three-dimensional graphene oxide framework (GOF) membrane for seawater desalination[J]. Desalination, 2016, 394: 123-130. DOI:10.1016/j.desal.2016.04.030 |
| [3] |
CHENG C, SHEN L, YU X, et al. Robust construction of a graphene oxide barrier layer on a nanofibrous substrate assisted by the flexible poly(vinylalcohol) for efficient pervaporation desalination[J]. Journal of Materials Chemistry A, 2017, 5(7): 3558-3568. DOI:10.1039/C6TA09443K |
| [4] |
HALAKOO E, FENG X. Layer-by-layer assembled membranes from graphene oxide and polyethyleneimine for ethanol and isopropanol dehydration[J]. Chemical Engineering Science, 2020, 216: 115488. DOI:10.1016/j.ces.2020.115488 |
| [5] |
SURI A, CALZAVARINI L, STRUNCK A B, et al. Comparison of chemical cross-linkers with branched and linear molecular structures for stabilization of graphene oxide membranes, and their performance in ethanol dehydration[J]. Industrial & Engineering Chemistry Research, 2019, 58(40): 18788-18797. |
| [6] |
KAMINSKI W, MARSZALEK J, TOMCZAK E. Water desalination by pervaporation-Comparison of energy consumption[J]. Desalination, 2018, 433: 89-93. DOI:10.1016/j.desal.2018.01.014 |
| [7] |
LIU G, JIN W, XU N. Graphene-based membranes[J]. Chemcal Society Review, 2015, 44(15): 5016-5030. DOI:10.1039/C4CS00423J |
| [8] |
LI X, ZHU B, ZHU J. Graphene oxide based materials for desalination[J]. Carbon, 2019, 146: 320-328. DOI:10.1016/j.carbon.2019.02.007 |
| [9] |
LERF A, BUCHSTEINER A, PIEPER J, et al. Hydration behavior and dynamics of water molecules in graphite oxide[J]. Journal of Physics and Chemistry of Solids, 2006, 67(5): 1106-1110. |
| [10] |
CERVENY S, BARROSO-BUJANS F, ALEGRíA Á, et al. Dynamics of water intercalated in graphite oxide[J]. The Journal of Physical Chemistry C, 2010, 114(6): 2604-2612. DOI:10.1021/jp907979v |
| [11] |
YANG Y, YANG X, LIANG L, et al. Large-area graphene-nanomesh/carbon-nanotube hybrid membranes for ionic and molecular nanofiltration[J]. Science, 2019, 364(6445): 1057-1062. DOI:10.1126/science.aau5321 |
| [12] |
SURWADE S P, SMIRNOV S N, VLASSIOUK I V, et al. Water desalination using nanoporous single-layer graphene[J]. Nature Nanotechnology, 2015, 10(5): 459-464. DOI:10.1038/nnano.2015.37 |
| [13] |
YEH C N, RAIDONGIA K, SHAO J, et al. On the origin of the stability of graphene oxide membranes in water[J]. Nature Chemistry, 2014, 7(2): 166-170. |
| [14] |
XI Y H, HU J Q, LIU Z, et al. Graphene oxide membranes with strong stability in aqueous solutions and controllable lamellar spacing[J]. ACS Applied Materials & Interfaces, 2016, 8(24): 15557-15566. |
| [15] |
ZHANG M, MAO Y, LIU G, et al. Molecular bridges stabilize graphene oxide membranes in water[J]. Angewandte Chemie, International Edition in English, 2020, 59(4): 1689-1695. DOI:10.1002/anie.201913010 |
| [16] |
QIAN Y, ZHOU C, HUANG A. Cross-linking modification with diamine monomers to enhance desalination performance of graphene oxide membranes[J]. Carbon, 2018, 136: 28-37. DOI:10.1016/j.carbon.2018.04.062 |
| [17] |
HUNG W-S, TSOU C-H, DE GUZMAN M, et al. Cross-linking with diamine monomers to prepare composite graphene oxide-framework membraneswith varying d-spacing[J]. Chemistry of Materials, 2014, 26(9): 2983-2990. DOI:10.1021/cm5007873 |
| [18] |
ZHANG Y, ZHANG S, CHUNG T S. Nanometric graphene oxide framework membranes with enhanced heavy metal removal via nanofiltration[J]. Environmental Science & Technology, 2015, 49(16): 10235-10242. |
| [19] |
DONG L, LI M, ZHANG S, et al. NH2-Fe3O4-regulated graphene oxide membranes with well-defined laminar nanochannels for desalination of dye solutions[J]. Desalination, 2020, 476: 114227. DOI:10.1016/j.desal.2019.114227 |
| [20] |
LIU S, HU K, CERRUTI M, et al. Ultra-stiff graphene oxide paper prepared by directed-flow vacuum filtration[J]. Carbon, 2020, 158: 426-434. DOI:10.1016/j.carbon.2019.11.007 |
| [21] |
HAN J L, HAIDER M R, LIU M J, et al. Borate inorganic cross-linked durable graphene oxide membrane preparation and membrane fouling control[J]. Environmental Science & Technology, 2019, 53(3): 1501-1508. |
| [22] |
JIA Z, WANG Y, SHI W, et al. Diamines cross-linked graphene oxide free-standing membranes for ion dialysis separation[J]. Journal of Membrane Science, 2016, 520: 139-144. DOI:10.1016/j.memsci.2016.07.042 |
| [23] |
PAN F, LI Y, SONG Y, et al. Graphene oxide membranes with fixed interlayer distance via dual crosslinkers for efficient liquid molecular separations[J]. Journal of Membrane Science, 2020, 595: 117486. DOI:10.1016/j.memsci.2019.117486 |
| [24] |
PARK S, LEE K S, BOZOKLU G, et al. Graphene oxide papers modified by divalent ions-enhancing mechanical properties via chemical cross-linking[J]. ACS Nano, 2008, 2(3): 572-578. DOI:10.1021/nn700349a |
| [25] |
GAO Y, SU K, WANG X, et al. A metal-nano GO frameworks/PPS membrane with super water flux and high dyes interception[J]. Journal of Membrane Science, 2019, 574: 55-64. DOI:10.1016/j.memsci.2018.12.052 |
| [26] |
CHEN L, SHI G, SHEN J, et al. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing[J]. Nature, 2017, 550(7676): 380-383. DOI:10.1038/nature24044 |
| [27] |
CHO K M, LEE H J, NAM Y T, et al. Ultrafast-selective nanofiltration of an hybrid membrane comprising laminated reduced graphene oxide/graphene oxide nanoribbons[J]. ACS Applied Materials & Interfaces, 2019, 11(30): 27004-27010. |
| [28] |
CHEN X, QIU M, DING H, et al. A reduced graphene oxide nanofiltration membrane intercalated by well-dispersed carbon nanotubes for drinking water purification[J]. Nanoscale, 2016, 8(10): 5696-5705. DOI:10.1039/C5NR08697C |
| [29] |
HAN Y, JIANG Y, GAO C. High-flux graphene oxide nanofiltration membrane intercalated by carbon nanotubes[J]. ACS Applied Materials & Interfaces, 2015, 7(15): 8147-8155. |
| [30] |
WEI Y, ZHANG Y, GAO X, et al. Multilayered graphene oxide membranes for water treatment: A review[J]. Carbon, 2018, 139: 964-981. DOI:10.1016/j.carbon.2018.07.040 |
| [31] |
刘阳, 顾平, 张光辉. 氧化石墨烯分离膜的制备及其水处理领域的应用进展[J]. 化工进展, 2017, 36(11): 4151-4159. LIU Y, GU P, ZHANG G H. Fabrication of graphene oxide-assisted membranes and its applications in water treatment and purification[J]. Chemical Industry and Engineering Progress, 2017, 36(11): 4151-4159. |
| [32] |
SUN J W, QIAN X W, WANG Z H, et al. Tailoring the microstructure of poly(vinyl alcohol)-intercalated graphene oxide membranes for enhanced desalination performance of high-salinity water by pervaporation[J]. Journal of Membrane Science, 2020, 599: 117838. DOI:10.1016/j.memsci.2020.117838 |
| [33] |
KRISHNAMOORTHY K, VEERAPANDIAN M, YUN K, et al. The chemical and structural analysis of graphene oxide with different degrees of oxidation[J]. Carbon, 2013, 53: 38-49. DOI:10.1016/j.carbon.2012.10.013 |
| [34] |
GUERRERO-CONTRERAS J, CABALLERO-BRIONES F. Graphene oxide powders with different oxidation degree, prepared by synthesis variations of the Hummers method[J]. Materials Chemistry and Physics, 2015, 153: 209-220. DOI:10.1016/j.matchemphys.2015.01.005 |
| [35] |
CHONG J Y, WANG B, LI K. Water transport through graphene oxide membranes: The roles of driving forces[J]. Chemical Communications, 2018, 54(20): 2554-2557. DOI:10.1039/C7CC09120F |
| [36] |
NAIR R R, WU H A, JAYARAM P N, et al. Unimpeded permeation of water through helium-leak-tight graphene-based membranes[J]. Science, 2012, 335(6067): 442-444. DOI:10.1126/science.1211694 |
| [37] |
LIANG B, LI Q, CAO B, et al. Water permeance, permeability and desalination properties of the sulfonic acid functionalized composite pervaporation membranes[J]. Desalination, 2018, 433: 132-140. DOI:10.1016/j.desal.2018.01.028 |
| [38] |
HUTH E, MUTHU S, RUFF L, et al. Feasibility assessment of pervaporation for desalinating high-salinity brines[J]. Journal of Water Reuse and Desalination, 2014, 4(2): 109-124. DOI:10.2166/wrd.2014.038 |
| [39] |
CHAUDHRI S G, CHAUDHARI J C, SINGH P S. Fabrication of efficient pervaporation desalination membrane by reinforcement of poly(vinyl alcohol)-silica film on porous polysulfone hollow fiber[J]. Journal of Applied Polymer Science, 2018, 135(3): 45718. DOI:10.1002/app.45718 |
| [40] |
NAIM M, ELEWA M, EL-SHAFEI A, et al. Desalination of simulated seawater by purge-air pervaporation using an innovative fabricated membrane[J]. Water Science and Technology, 2015, 72(5): 785-793. DOI:10.2166/wst.2015.277 |
| [41] |
CHAUDHRI S G, RAJAI B H, SINGH P S. Preparation of ultra-thin poly(vinyl alcohol) membranes supported on polysulfone hollow fiber and their application for production of pure water from seawater[J]. Desalination, 2015, 367: 272-284. DOI:10.1016/j.desal.2015.04.016 |
| [42] |
LI Q, CAO B, LI P. Fabrication of high performance pervaporation desalination composite membranes by optimizing the support layer structures[J]. Industrial & Engineering Chemistry Research, 2018, 57(32): 11178-11185. |
| [43] |
ZHANG R, LIANG B, QU T, et al. High-performance sulfosuccinic acid cross-linked PVA composite pervaporation membrane for desalination[J]. Environmental Technology, 2019, 40(3): 312-320. DOI:10.1080/09593330.2017.1388852 |
| [44] |
LIU G, SHEN J, LIU Q, et al. Ultrathin two-dimensional MXene membrane for pervaporation desalination[J]. Journal of Membrane Science, 2018, 548: 548-558. DOI:10.1016/j.memsci.2017.11.065 |
| [45] |
XU K, FENG B, ZHOU C, et al. Synthesis of highly stable graphene oxide membranes on polydopamine functionalized supports for seawater desalination[J]. Chemical Engineering Science, 2016, 146: 159-165. DOI:10.1016/j.ces.2016.03.003 |
| [46] |
QIAN X, LI N, WANG Q, et al. Chitosan/graphene oxide mixed matrix membrane with enhanced water permeability for high-salinity water desalination by pervaporation[J]. Desalination, 2018, 438: 83-96. DOI:10.1016/j.desal.2018.03.031 |
| [47] |
LIANG B, ZHAN W, QI G, et al. High performance graphene oxide/polyacrylonitrile composite pervaporation membranes for desalination applications[J]. Journal of Materials Chemistry A, 2015, 3(9): 5140-5147. DOI:10.1039/C4TA06573E |
| [48] |
HALAKOO E, FENG X. Layer-by-layer assembly of polyethyleneimine/graphene oxide membranes for desalination of high-salinity water via pervaporation[J]. Separation and Purification Technology, 2020, 234: 116077. DOI:10.1016/j.seppur.2019.116077 |
| [49] |
QIAN Y, ZHANG X, LIU C, et al. Tuning interlayer spacing of graphene oxide membranes with enhanced desalination performance[J]. Desalination, 2019, 460: 56-63. DOI:10.1016/j.desal.2019.03.009 |
| [50] |
LI L, HOU J, CHEN V. Pinning down the water transport mechanism in graphene oxide pervaporation desalination membranes[J]. Industrial & Engineering Chemistry Research, 2019, 58(10): 4231-4239. |
| [51] |
YANG G, XIE Z, CRAN M, et al. Functionalizing graphene oxide framework membranes with sulfonic acid groups for superior aqueous mixture separation[J]. Journal of Materials Chemistry A, 2019, 7(34): 19682-19690. DOI:10.1039/C9TA04031E |






