2. 浙江工业大学 土木工程学院, 浙江 杭州 310014;
3. 厦门市水资源利用与保护重点实验室, 福建 厦门 361024
2. College of Civil Engineering and Architecture, Zhejiang University of Technology, Hangzhou 310014, China;
3. Key Laboratory of Water Resources Utilization and Protection, Xiamen City, Xiamen 361024, China
水环境中药品和个人护理品(pharmaceuticals and personal care products,PPCPs)对人体健康的危害引起了广泛关注。据报道,水体中PPCPs检出频次日益增多[1-3],因此有必要对其进行去除。研究表明O3氧化[4]、电化学氧化[5]、紫外协同TiO2(UV/TiO2)[6]、UV/H2O2[7]和UV/PS[8]等工艺可以有效去除水环境中PPCPs,其中UV/氯工艺被认为是处理受污染水(饮用水和再生水)的一个高效选择[9]。UV/NaClO工艺可产生羟基自由基(HO·)、氯自由基(Cl·)、二氯自由基(Cl2−·)、氯氧自由基(ClO·)、氧自由基(O−·)及次氯酸根自由基(ClOH·)等多种氧化性自由基[10-11]。HO·可以无选择地降解有机污染物,而含氯自由基(RCS)则对于含芳香环和富电子结构的污染物具有选择性的去除。不同自由基(HO·、RCS)在UV/NaClO工艺中的贡献取决于自由基的浓度和目标污染物的结构特性[12~15],因而自由基的浓度及其与目标物的反应速率常数是控制污染物去除效能的重要因素[16]。目前,关于UV/NaClO工艺组分贡献的研究中自由基常被作为研究重点[17~18],但反应涉及自由基种类繁多,去除机理复杂,其中Cl·和ClO·由于缺少针对性的淬灭剂及探针物质,竞争动力学降解实验中常被简单归为RCS[19],导致其贡献难以准确评价。因此需要进一步研究Cl·和ClO·的浓度及其与目标污染物的反应速率常数,探明HO·、Cl·和ClO·等组分在UV/NaClO工艺降解污染物过程中的贡献。
邻苯基苯酚(o-phenylphenol,OPP)是一种典型酚类PPCPs,常被添加于杀菌剂和防腐剂中,在地表水和污水污泥中均有检出[20]。OPP具有一定的危害[21],2017年被世界卫生组织列为致癌物[22]。紫外发光二极管(UV-LED)灯作为一种新兴的半导体技术逐渐成为基于UV的高级氧化工艺中的紫外光源。相对于传统UV灯,UV-LED有独特的优势:(1) 安全无毒;(2) 灯珠寿命长,不易损坏;(3) 无需提前预热,安全可控。因此UV-LED在水处理领域中有替换传统低压汞灯的潜力。
本研究以OPP为目标污染物,采用紫外发光二极管(UV-LED)/NaClO工艺对其去除进行研究。利用自由基淬灭剂和探针物质组合建立竞争动力学实验,确定了UV-LED/NaClO工艺中HO·、Cl·和ClO·浓度及其与OPP二级反应速率常数,对比了不同组分(UV-LED光解、NaClO氯化和自由基氧化等)对OPP去除的相对贡献,考察了NaClO投加量和pH值对OPP降解过程中不同组分贡献的影响。以期为UV-LED/NaClO工艺降解水环境中微量污染物提供理论支撑和基础数据。
2 实验(材料与方法) 2.1 实验试剂与仪器OPP(德国Dr.Ehrenstorfer公司,质量分数w > 99.9%);沙丁胺醇(salbutamol,SAL)(德国Dr.Ehrenstorfer公司,w > 99.8%);C2H7NO2、KH2PO4 (HPLC级,中国aladdin);乙腈(HPLC级,德国Merck)、硝基苯(nitrobenzene,NB)、苯甲酸(benzoic acid,BA)、对二甲氧基苯(1,4-dimethoxybenzene,DMOB)、叔丁醇(tert-butanol,TBA)(HPLC级,上海安谱);次氯酸钠(CP,活性氯质量分数≥5.2%);Na2S2O3·5H2O、K2HPO4·3H2O、NaHCO3、HCl和NaOH均为分析纯;实验室用水均为Mill-Q超纯水(电阻≤18.2 MΩ)。
LC-20A高效液相色谱仪(Shimadzu,日本);DZF-6050真空干燥箱(上海精宏实验设备有限公司),纯水机(Milipore,美国),HJ-6A型磁力恒温搅拌器(江苏金坛峥嵘仪器),pH计(Eutevch,美国),UV-LED灯(深圳微紫科技有限公司,波长278 nm,单个100 mW)。
2.2 实验方法实验于放置在磁力搅拌器上的石英筒管(300 mL)中进行,筒管中添加由磷酸盐缓冲液配制的OPP溶液(浓度为3.0 μmol⋅L−1,pH=7.0±0.2)。考察初始pH的影响时通过0.1 mol⋅L−1的HCl或NaOH调节溶液pH为设定值。光源采用8个UV-LED小灯珠(光强为0.33 mW∙cm−2)并联组成,内置于石英套管中。实验时先投加一定量的NaClO溶液(1∼7 mg⋅L−1),然后启动UV-LED灯开始实验。设定时间取出3 mL水样,经0.1 mol⋅L−1的Na2S2O3淬灭过膜后,采用HPLC测定溶液中剩余OPP浓度。所有条件下的反应均重复3次,取平均值。
本研究采用自由基探针来确定自由基浓度和二级反应速率常数。由于UV-LED/H2O2工艺中仅生成HO·,因此采用NB作为HO·的探针化合物,通过竞争动力学计算出HO·的浓度及其与OPP的二级反应速率常数。在UV-LED/NaClO工艺中,利用TBA和NaHCO3组合淬灭HO·、Cl·、ClO·及Cl2−·,再加入SAL排除CO3−·的影响,计算出ClO·与OPP的二级反应速率常数。将计算出的二级反应速率常数代入含NB、BA、DMOB的竞争动力学公式,可求得UV-LED/NaClO工艺中HO·、Cl·和ClO·的稳态浓度及其与OPP的二级反应速率常数。
2.3 分析方法OPP、NB、SAL、BA和DMOB浓度采用HPLC进行测定。检测波长为分别为254、262、223、230和225 nm。流动相是体积比为60:40的乙腈和水(SAL的水相为7.5 mmol⋅L−1的KH2PO4溶液,BA的水相为0.02 mmol⋅L−1的C2H7NO2溶液),流速均为1.0 mL⋅min−1,柱温设定为40 ℃,进样量为10 μL。
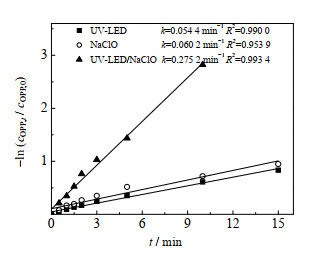
3 结果与讨论 3.1 UV-LED、NaClO和UV-LED/NaClO对OPP的去除对比考察了UV-LED、NaClO和UV-LED/NaClO对OPP的去除,结果如图 1所示。cOPP, 0为OPP的初始浓度,cOPP, t为OPP在t时刻的浓度,ρNaClO为NaClO的质量浓度。
|
图 1 OPP在UV-LED、NaClO和UV-LED/NaClO工艺中的降解动力学曲线 Fig.1 OPP degradation kinetic curves under UV-LED irradiation, NaClO oxidation and UV-LED/NaClO processes cOPP, 0=3 μmol·L-1, ρNaClO =3 mg·L-1, pH=7.0±0.2 |
实验反应900 s后UV-LED和NaClO对OPP的去除分别为56.6% 和61.3%,而同样时间内UV-LED/NaClO对OPP的去除可达100%。拟合UV-LED辐射、NaClO氧化和UV-LED/NaClO工艺的降解曲线,其拟一级动力学常数k分别为0.054 4、0.060 2和0.275 2 min−1。联用工艺降解OPP的拟一级动力学常数分别为UV-LED和NaClO的5倍和4倍,UV-LED/NaClO工艺对OPP的降解有协同作用。
UV-LED对OPP的去除原因可能是由于OPP受UV-LED照射后被直接光解。NaClO通过氧化作用对OPP也有明显的去除。UV-LED与NaClO联用显著提高了OPP的去除,这是因为溶液中的HOCl/OCl−在UV-LED的辐射下生成大量的HO·和RCS等氧化性自由基[23-29],这些自由基可通过单电子氧化、脱H+、C─C不饱和键加成等途径有效去除OPP[30-32],主要反应式为式(1)~(6)。Pan等[24]、Xiong等[33]采用UV/NaClO降解甲硝哒唑和普萘诺尔时发现UV与NaClO有明显协同作用,与本研究结论类似。
| $ \mathrm{HClO} / \mathrm{OCl}^{-}+\mathrm{hv} \rightarrow \mathrm{HO} \cdot+\mathrm{Cl}\cdot $ | (1) |
| $ \mathrm{Cl}^{-}+\mathrm{Cl} \cdot \underset{k_2}{\stackrel{k_1}{\rightleftarrows}} \mathrm{Cl}_2^{-} \cdot\left(k_1=6.5 \times 10^9 \mathrm{~L} \cdot \mathrm{mol}^{-1} \cdot \mathrm{s}^{-1}, k_2=1.1 \times 10^5 \mathrm{~L} \cdot \mathrm{mol}^{-1} \cdot \mathrm{s}^{-1}\right) $ | (2) |
| $ \mathrm{HClO}+\mathrm{HO} \cdot \stackrel{k_3}{\longrightarrow} \mathrm{H}_2 \mathrm{O}+\mathrm{ClO}\cdot \quad\left(k_3=2.0 \times 10^9 \mathrm{~L} \cdot \mathrm{mol}^{-1} \cdot \mathrm{s}^{-3}\right) $ | (3) |
| $ \mathrm{ClO}^{-}+\mathrm{HO} \cdot \stackrel{k_4}{\longrightarrow} \mathrm{OH}^{-}+\mathrm{ClO}\cdot\quad\left(k_4=8.8 \times 10^9 \mathrm{~L} \cdot \mathrm{mol}^{-1} \cdot \mathrm{s}^{-1}\right) $ | (4) |
| $ \mathrm{Cl} \cdot+\mathrm{HClO} \stackrel{k_5}{\longrightarrow} \mathrm{H}^{+}+\mathrm{Cl}^{-}+\mathrm{ClO}\cdot \quad\left(k_5=3.0 \times 10^9 \mathrm{~L} \cdot \mathrm{mol}^{-1} \cdot \mathrm{s}^{-1}\right) $ | (5) |
| $ \mathrm{Cl} \cdot+\mathrm{ClO}^{-} \stackrel{k_6}{\longrightarrow} \mathrm{Cl}^{-}+\mathrm{ClO}\cdot \quad\left(k_6=8.2 \times 10^9 \mathrm{~L} \cdot \mathrm{mol}^{-1} \cdot \mathrm{s}^{-1}\right) $ | (6) |
HO·、Cl·和ClO·等自由基对污染物去除有不同的贡献[32]。为了进一步探究HO·、Cl·及ClO·等自由基对OPP去除贡献及其与OPP的反应速率,采用竞争动力学模型对其进行研究。
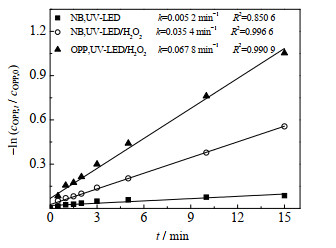
NB与HO·发生专一性反应(二级反应速率常数为3.9×109 L⋅mol−1⋅s−1),H2O2在UV-LED照射下仅产生HO·[25, 34]。UV-LED/H2O2工艺对NB和OPP的降解如图 2所示。由图 1、2可知,单独UV-LED对NB和OPP的拟一级降解速率常数分别为0.005 2和0.054 4 min−1,此外实验中发现单独H2O2无法去除NB和OPP。NB和OPP的去除则可用式(7)和(8)来表示。
|
图 2 NB和OPP在UV-LED/H2O2工艺中的降解动力学曲线 Fig.2 Degradation kinetic curves of NB and OPP under UV-LED/H2O2 processes cOPP, 0=3μmol·L-1, cNB, 0=3μmol·L-1, ρH2O2=10 mg·L-1, pH=7.0±0.2 |
| $ k_{\mathrm{NB}}=k_{\mathrm{UV}, \mathrm{LED}, \mathrm{NB}}+k_{\mathrm{HO} \cdot, \mathrm{NB}} c_{\mathrm{HO} \cdot, \mathrm{ss}} $ | (7) |
| $ k_{\mathrm{OPP}}=k_{\mathrm{UV}, \mathrm{LED}, \mathrm{OPP}}+k_{\mathrm{HO}\cdot, \mathrm{OPP}} c_{\mathrm{HO}\cdot, \mathrm{ss}} $ | (8) |
式中:
由图 2可知,UV-LED/H2O2降解NB和OPP的拟一级动力学常数为0.035 4及0.067 8 min−1。由式(7)和(8)计算出kHO·, OPP=1.86×109 L⋅mol−1⋅s−1。OPP为芳香环化合物,Lei等[35]研究表明含芳香环结构的PPCPs与HO·速率常数为108 ~1010 L⋅mol−1⋅s−1,实验中OPP与HO·的反应速率与此范围相符。
3.2.2 Cl·、ClO·与OPP的二级反应速率常数TBA可与HO·、Cl·和ClO·反应,常用于测定AOPs中RCS的贡献[30-31]。NaHCO3能有效淬灭HO·、Cl·及Cl2−·。UV-LED/NaClO工艺投加TBA和NaHCO3可以淬灭相应自由基。但NaHCO3会消耗HO·和Cl·生成CO3−· (式(9)和(10))。
| $ \mathrm{HO} \cdot+\mathrm{HCO}_3^{-} \stackrel{k_7}{\longrightarrow} \mathrm{H}_2 \mathrm{O}+\mathrm{CO}_3^{-} \cdot \quad\left(k_7=8.5 \times 10^6 \mathrm{~L} \cdot \mathrm{mol}^{-1} \cdot \mathrm{s}^{-1}\right) $ | (9) |
| $ \mathrm{Cl} \cdot+\mathrm{HCO}_3^{-} \stackrel{k_8}{\longrightarrow} \mathrm{Cl}^{-}+\mathrm{CO}_3^{-} \cdot \quad\left(k_8=5.0 \times 10^8 \mathrm{~L} \cdot \mathrm{mol}^{-1} \cdot \mathrm{s}^{-1}\right) $ | (10) |
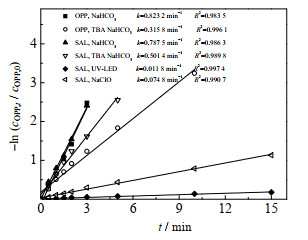
CO3−·是一种高选择性自由基(氧化还原电位E0=1.78 V),与含富电子基团的化合物及苯酚、苯胺等含芳香环结构的物质反应具有较高的反应速率[36-37]。实验中HCO3−可明显促进OPP的降解,为排除CO3−·的影响,UV-LED/NaClO工艺中添加TBA、NaHCO3、SAL和OPP进行竞争动力学实验,结果如图 3所示。SAL与HO·、ClO·和CO3−·的二级反应速率常数kHO·, SAL=2.62×109 L⋅mol−1⋅s−1、kClO·, SAL=1.21×108 L⋅mol−1⋅s−1及kCO3·, SAL−=2.29×108 L⋅mol−1⋅s−1 [34-38]。UV-LED和NaClO降解SAL的拟一级动力学常数为0.011 8 min−1。单独投加NaHCO3时,OPP和SAL的降解动力学可表示为式(11)和(12)。
|
图 3 OPP和SAL在含TBA和NaHCO3的UV-LED/NaClO工艺中的降解动力学曲线 Fig.3 Degradation kinetic curves of OPP and SAL under UV-LED/NaClO processes containing TBA and NaHCO3 cOPP, 0=3 μmol·L-1, cSAL, 0=3 μmol·L-1, ρNaClO=3 mg·L-1, cTBA=100 mmol·L-1, cNaHCO3=50 mmol·L-1, pH=7.0±0.2 |
| $ -\ln \frac{c_{\mathrm{OPP}, t}}{c_{\mathrm{OPP}, 0}}=k_{1, \mathrm{obs}}=k_{\mathrm{UV}-\mathrm{LED}, \mathrm{OPP}}+k_{\mathrm{NaClO}, \mathrm{OPP}}+k_{\mathrm{ClO·}, \mathrm{OPP}} c_{\mathrm{ClO·}, \mathrm{ss}}+k_{\mathrm{CO}_3^{-}·, \mathrm{opp}} c_{\mathrm{CO}_3^{-}·, \mathrm{ss}}+k_{\mathrm{other}, \mathrm{OPP}} $ | (11) |
| $ -\ln \frac{c_{\mathrm{SAL}, t}}{c_{\mathrm{SAL}, 0}}=k_{2, \mathrm{obs}}=k_{\mathrm{UV}-\mathrm{LED}, \mathrm{SAL}}+k_{\mathrm{NaClO}, \mathrm{SAL}}+k_{\mathrm{ClO·}, \mathrm{SAL}} c_{\mathrm{ClO·}, \mathrm{ss}}+k_{\mathrm{CO}_3^{-}·, \mathrm{SAL}} c_{\mathrm{CO}_3^{-}·, \mathrm{ss}}+k_{\text {other,SAL }} $ | (12) |
式中:kother, OPP和koher, SAL为其他未被淬灭的活性组分对OPP和SAL去除的拟一级动力学速率常数;cClO·, ss和cCO3·, ss−为ClO·和CO3−·在溶液中的稳态浓度;kUV-LED, SAL和kUV-LED, OPP为单独UV-LED降解SAL和OPP的拟一级动力学常数;kNaClO, SAL和kNaClO, OPP表示单独NaClO降解SAL和OPP的拟一级动力学常数。
| $ -\ln \frac{c_{\mathrm{OPP}, t}}{c_{\mathrm{OPP}, 0}}=k_{3, \mathrm{obs}}=k_{\mathrm{UV}-\mathrm{LED}, \mathrm{OPP}}+k_{\mathrm{NaClO}, \mathrm{OPP}}+k_{\mathrm{CO}_3^{-}·, \mathrm{OPP}} c_{\mathrm{CO}_3^{-}·, \mathrm{ss}}+k_{\text {other,OPP }} $ | (13) |
| $ -\ln \frac{c_{\mathrm{SAL}, t}}{c_{\mathrm{SAL}, 0}}=k_{4, \mathrm{obs}}=k_{\mathrm{UV}-\mathrm{LED}, \mathrm{SAL}}+k_{\mathrm{NaClO}, \mathrm{SAL}}+k_{\mathrm{CO}_3^{-}·, \mathrm{SAL}} c_{\mathrm{CO}_3^{-}·, \mathrm{ss}}+k_{\text {other, } \mathrm{SAL}} $ | (14) |
式中:k1, obs、k2, obs、k3, obs、k4, obs分别为SAL和OPP在不同条件下的拟一级动力学常数,分别为0.823 2、0.787 5、0.315 8和0.501 4 min−1。通过式(11)~(14)则可推出式(15)和(16)
| $ c_{\mathrm{ClO} \cdot, \mathrm{ss}}=\frac{k_{2, \mathrm{obs}}-k_{4, \mathrm{obs}}}{k_{\mathrm{ClO} \cdot, \mathrm{SAL}}} $ | (15) |
| $ k_{\mathrm{ClO} \cdot, \mathrm{OPP}}=\frac{k_{1, \mathrm{obs}}-k_{3, \mathrm{obs}}}{c_{\mathrm{ClO} \cdot, \mathrm{ss}}} $ | (16) |
由式(15)和(16)可求出cClO·, ss=3.88×10−11 mol⋅L−1,kClO·, OPP= 2.18×108 L⋅mol−1⋅s−1。Yang等[39]采用竞争动力学计算出苯并噻唑和苯并三唑与ClO·的二级反应速率常数分别为2.22×108和2.40×108 L⋅mol−1⋅s−1,其速率常数略大于本实验中的kClO·, OPP,但仍为同一数量级。
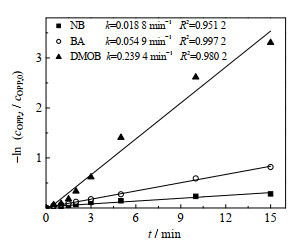
采用NB、BA和DMOB作为探针化合物,NB、BA和DMOB在UV-LED/NaClO工艺中的去除如图 4所示。
|
图 4 NB、BA和DMOB在UV-LED/NaClO工艺中的降解动力学曲线 Fig.4 Degradation kinetic curves of NB, BA and DMOB under UV-LED/NaClO processes cOPP, 0=3 μmol·L-1, cNB, 0=3 μmol·L-1, cBA, 0=3 μmol·L-1, cDMOB, 0=3 μmol·L-1, ρNaClO=3 mg·L-1, pH=7.0±0.2 |
DMOB可以与HO·、Cl·和ClO·进行反应,其二级反应速率常数分别为7.0×109、1.8×1010及2.1×109 L⋅mol−1⋅s−1,而BA与ClO·不发生反应,其与HO·、Cl·的二级反应速率常数为5.9×109和1.8×1010 L⋅mol−1⋅s−1 [25]。单独UV-LED和NaClO无法降解BA和DMOB[25],其反应式如式(17)~(19)。
| $ -\ln \frac{c_{\mathrm{NB}, t}}{c_{\mathrm{NB}, 0}}=k_{\mathrm{UV}-\mathrm{LED}, \mathrm{NB}}+k_{\mathrm{HO} \cdot, \mathrm{NB}} c_{\mathrm{HO}\cdot, \mathrm{ss}} $ | (17) |
| $ -\ln \frac{c_{\mathrm{BA}, t}}{c_{\mathrm{BA}, 0}}=k_{\mathrm{HO}, \mathrm{BA}} c_{\mathrm{HO} \cdot, \mathrm{ss}}+k_{\mathrm{Cl}\cdot, \mathrm{BA}} c_{\mathrm{Cl·}, \mathrm{ss}} $ | (18) |
| $ -\ln \frac{c_{\mathrm{DMOB}, t}}{c_{\mathrm{DMOB}, 0}}=k_{\mathrm{HO} \cdot, \mathrm{DMOB}} c_{\mathrm{HO·}, \mathrm{ss}}+k_{\mathrm{Cl·}, \mathrm{DMOB}} c_{\mathrm{Cl·}, \mathrm{ss}}+k_{\mathrm{ClO·}, \mathrm{DMOB}} c_{\mathrm{ClO·}, \mathrm{ss}} $ | (19) |
式中:kUV-LED, NB表示单独UV-LED降解NB的拟一级动力学常数;kHO·, NB表示NB与HO·的二级反应速率常数;kHO·BA、kCl·, BA表示BA与HO·、BA与Cl·的二级反应速率常数;kHO·, DMOB、kCl·, DMOB、kClO·, DMOB表示DMOB与HO·、DMOB与Cl·、DMOB与ClO·的二级反应速率常数。利用上式计算可得到UV-LED/NaClO工艺中HO·、Cl·及ClO·的稳态浓度分别为1.22×10−13、1.08×10−14及1.38×10−12 mol⋅L−1。
OPP的去除可用式(20)表示:
| $ k_{\mathrm{obs}, \mathrm{OPP}}=k_{\mathrm{UV}-\mathrm{LED}, \mathrm{OPP}}+k_{\mathrm{NaClO}, \mathrm{OPP}}+k_{\mathrm{HO} \cdot, \mathrm{OPP}} c_{\mathrm{HO} \cdot, \mathrm{ss}}+k_{\mathrm{Cl·}, \mathrm{OPP}} c_{\mathrm{Cl·}, \mathrm{ss}}+k_{\mathrm{ClO·}, \mathrm{OPP}} c_{\mathrm{ClO·}, \mathrm{ss}}+k_{\mathrm{other}, \mathrm{OPP}} $ | (20) |
式中:kUV-LED, OPP和kNaClO, OPP表示单独UV-LED和单独NaClO降解OPP的拟一级动力学常数;kobs, OPP表示UV-LED/NaClO降解OPP的拟一级动力学常数;kother表示其他组分(Cl2−·、O−·、ClOH·和水合电子eaq等)对OPP的降解贡献。TBA对其他组分无淬灭效果,其贡献可用式(21)表示。
| $ k_{\mathrm{other}, \mathrm{OPP}}=k_{\mathrm{obs}, \mathrm{TBA}} $ | (21) |
由式(20)和(21)计算得到kCl·, OPP=1.33×1011 L⋅mol−1s−1。Cl·与OPP反应速率最快,HO·次之,ClO·与OPP的反应速率最小,由此可知增大Cl·的浓度可以提高OPP的去除。研究表明卡马西平和氟康唑与Cl·的二级速率常数均大于其与HO·的速率常数[16, 40],本实验具有类似的结论,这可能是因为3种污染物皆属芳香环化合物,HO·和Cl·都能将其去除,但Cl·的选择性导致其与含芳香环的物质反应更快,因此3种污染物去除过程中其与Cl·的二级反应速率常数大于HO·。
3.3 UV-LED/NaClO降解OPP过程中不同组分的贡献基于上述实验中已确定HO·、Cl·和ClO·与OPP的二级反应速率常数、自由基稳态浓度,kother的贡献值可通过式(21)计算得到。为了考察不同组分对OPP降解的贡献,采用OPP、NB、BA和DMOB的混合竞争动力学模型对其进行研究,通过拟一级动力学常数确定降解过程中不同组分的相对贡献,可用式(20)表示,结果如图 5所示。NaClO、UV-LED、HO·、ClO·、Cl·和其他组分对OPP降解的贡献率分别为22.17%、19.15%、5.01%、6.44%、31.13% 和16.1%。活性自由基(HO·、ClO·和Cl·)对OPP的去除贡献占58.56%,贡献值HO· < ClO· < Cl·,其中Cl·贡献占自由基总贡献的53.16%,这可能是因为HO·与ClO·二级反应速率常数远小于Cl·,导致HO·与ClO·对OPP的降解贡献仅为11.45%,低于单独Cl·贡献。
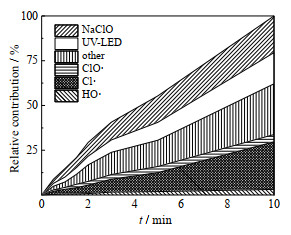
|
图 5 UV-LED/NaClO工艺中不同组分对OPP降解的贡献 Fig.5 Effects of different species on OPP degradation under UV-LED/NaClO processes cOPP, 0=3 μmol·L-1, ρNaClO=3 mg·L-1, pH=7.0±0.2 |
RCS与UV-LED对OPP降解起重要作用[41],其对OPP的降解贡献为56.72%,其中Cl·和UV-LED的相对降解贡献占主要部分,这是因为一方面由于Cl·氧化还原电位高于ClO·[42],与OPP反应速率高于HO·;另一方面OPP摩尔吸光系数为4 620 L⋅mol−1⋅cm−1(紫外波长=278 nm),光量子产率φ为0.001 9(pH=7时)[43],吸收UVC后易被直接光解。这与Kong等[44]采用UV/氯工艺降解丁二烯时发现UV和RCS相对贡献远大于HO·的结论类似。实际水体中由于天然有机物(NOM)和阴离子(Br−、NO2−和I−等)的存在,会抑制紫外/氯工艺中活性组分(HO·和RCS)的产生,降低OPP的去除[45]。
3.4 pH与NaClO投加量对UV-LED/NaClO工艺去除OPP的影响 3.4.1 pH对不同组分去除OPP贡献的影响pH影响UV-LED/NaClO工艺中活性基团的浓度和存在形式[46],因此考察了pH对OPP降解中各组分贡献的影响,结果如图 6所示。
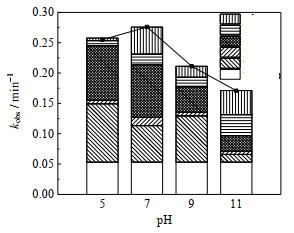
|
图 6 pH值对UV-LED/NaClO去除OPP的影响 Fig.6 Effects of pH on OPP degradation under UV-LED/NaClO processes cOPP, 0=3 μmol·L-1, ρNaClO =3 mg·L-1, pH=7.0±0.2 |
不同pH值时OPP的降解均符合拟一级动力学模型(R2 > 0.97),当pH值为5、7、9和11时,OPP降解的拟一级动力学速率常数分别为0.255 0、0.275 2、0.211 2和0.171 0 min−1,降解速率常数随pH值的增大先增加后减少。中性时有利于OPP的去除。实验中Cl·的贡献率随pH值增加由35.29% 降到15.05%,而ClO·贡献率则从3.29% 增大至20.07%,HO·的贡献几乎不受pH值的影响。这是因为一方面碱性溶液中OCl−浓度增大,而OCl−会与HO·及Cl·反应生成氧化电位较低的Cl2−·[46]。另一方面pH增大使溶液中Cl·被H+以及低电位的eaq大量消耗,而少部分氯会因磁力搅拌作用形成Cl2挥发[44],导致溶液中Cl·浓度降低,与OPP反应速率较低的ClO·取代Cl·成为OPP降解的主要活性组分(式(5)、(22)和(23)),同时OPP的量子产率随pH升高而降低[41],因而碱性环境对OPP的降解起抑制作用。中性环境下,溶液中的HOCl浓度增大,与OCl−反应生成更多的HO·和Cl·,进而更有效地去除OPP。溶液呈酸性时,Cl·浓度增大,但eaq与H+以2.4×109 L⋅mol−1⋅s−1的速率相互消耗[47],导致其他组分贡献显著减少,因此溶液在pH=5时降解速率略低于pH=7时。李博强等[48]在研究UV-LED/NaClO去除AAP时得到类似降解规律,AAP在中性环境中降解效果最好。Kim等[49]研究UV-LED/NaClO工艺降解红霉素时,发现ClO·的贡献随着pH的增加而增加,而Cl·贡献随着pH的增加而减少,结论与本研究具有同样的结论。然而Kong等[50]使用UV/NaClO工艺降解吉非贝齐时发现HO·的贡献随pH增大而增加,RCS的贡献随pH增大而减少。这可能是因为RCS浓度增大时其高选择性导致对污染物去除效果增加不明显,从而使贡献减小,但本实验并未观察到这一现象。
| $ \mathrm{Cl} \cdot+e_{\mathrm{aq}}+\mathrm{H}^{+} \longrightarrow \mathrm{H} \cdot+\mathrm{Cl}^{-} $ | (22) |
| $ \mathrm{Cl}_2^{-} \cdot+\mathrm{Cl}_2^{-} \cdot \stackrel{k_9}{\longrightarrow} 2 \mathrm{Cl}^{-}+\mathrm{Cl}_2 \quad\left(k_9=9.8 \times 10^8 \mathrm{~L} \cdot \mathrm{mol}^{-1} \cdot \mathrm{s}^{-1}\right) $ | (23) |
氧化剂的投加量对AOPs的去除污染物的性能有着重要的影响[51]。因此考察了NaClO投加量对OPP去除的影响(见图 7)。
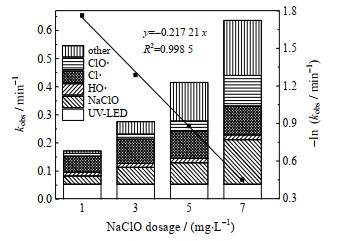
|
图 7 NaClO投加量对UV-LED/NaClO下不同组分去除OPP贡献的影响 Fig.7 Effects of different species on OPP degradation under UV-LED/NaClO processes at different NaClO concentrations cOPP, 0=3 μmol·L-1, pH=7.0±0.2 |
由图 7可知,当NaClO质量浓度为1、3、5及7 mg⋅L−1时,OPP降解的拟一级动力学常数为0.171 6、0.275 7、0.415 2及0.637 2 min−1。同时,OPP的降解速率常数与NaClO的投加量呈线性关系(R2=0.998 5),UV-LED/NaClO工艺中OPP的降解速率常数随着NaClO投加量增大而增大,这是因为HO·和RCS的浓度随着NaClO浓度增加而增多[25],NaClO的氧化去除效果也因投加量增加而去除更多的OPP,这与Gao等[52]研究UV-LED/NaClO工艺降解碘帕醇时所得结论相同。
当NaClO的质量浓度为1、3、5及7 mg⋅L−1时,HO·对OPP的降解贡献率分别为7.62%、5.01%、3.79% 及2.69%,Cl·对OPP的降解贡献率为33.50%、31.13%、27.19% 及16.10%,ClO·对OPP的降解贡献率为5.87%、6.44%、8.63% 及17.04%。HO·、Cl·的相对贡献随NaClO浓度增高而减小,ClO·的相对贡献则随之增大。这是由于当NaClO浓度增大时,溶液中HOCl/OCl−增多,加快与HO·和Cl·的反应生成大量ClO·,如式(3)~(6),导致HO·和Cl·浓度增幅变小,ClO·浓度增幅变大,因此其相对贡献也随之变化。Wu等[53]采用UV/NaClO降解CAF和NDA时发现Cl·浓度增量会随着NaClO投加量增加而减小,ClO·则会随之增大,结论与本实验类似。
4 结论(1) UV-LED/NaClO工艺对OPP的去除符合拟一级动力学(k=0.275 2 min−1),UV-LED/NaClO工艺能有效去除OPP。
(2) Cl·与OPP反应最快,HO·次之,ClO·与OPP反应最慢。Cl·、HO·和ClO·与OPP的二级反应速率常数分别为1.86×109、1.33×1011和2.18×108 L⋅mol−1⋅s−1,其稳态浓度分别为1.22×10−13、1.08×10−14及1.38×10−12 mol⋅L−1。
(3) UV-LED/NaClO工艺降解OPP过程中,不同组分贡献依次为:Cl· > NaClO > UV-LED > 其他组分 > HO· > ClO·,自由基是主要贡献组分,占总体贡献53.16%。
(4) 中性环境能够促进UV-LED/ NaClO工艺中OPP降解。酸性和中性环境中Cl·对OPP的降解起主要作用,而碱性环境中ClO·起主要作用。OPP降解速率常数和ClO·的贡献率随NaClO投加量的增大而增大,而HO·和Cl·的贡献率则随NaClO投加量的增大而减小。
| [1] |
王丹丹, 张婧, 杨桂朋, 等. 药物及个人护理品的污染现状、分析技术及生态毒性研究进展[J]. 环境科学研究, 2018, 31(12): 2013-2020. WANG D D, ZHANG J, YANG G P, et al. Pollution status, analytical techniques and ecotoxicity of pharmaceuticals and personal care products (PPCPs)[J]. Research of Environmental Sciences, 2018, 31(12): 2013-2020. DOI:10.13198/j.issn.1001-6929.2018.10.04 |
| [2] |
DAUGHTON C G. Cradle-to-cradle stewardship of drugs for minimizing their environmental disposition while promoting human health. II. Drug disposal, waste reduction, and future directions[J]. Environmental Health Perspectives, 2003, 111(5): 775-785. DOI:10.1289/ehp.5948 |
| [3] |
李超予, 杨怡潇, 张宁, 等. 两种典型PPCPs在潜流人工湿地中的季节性去除效果及降解产物[J]. 环境科学, 2021, 42(2): 842-849. LI C Y, YANG Y X, ZHANG N, et al. Seasonal removal efficiency and degradation products of two typical PPCPs in subsurface flow constructed wetlands[J]. Environmental Science, 2021, 42(2): 842-849. DOI:10.13227/j.hjkx.202004037 |
| [4] |
LIU C, NANABOINA V, KORSHIN G V, et al. Spectroscopic study of degradation products of ciprofloxacin, norfloxacin and lomefloxacin formed in ozonated wastewater[J]. Water Research, 2012, 46(16): 5235-5246. DOI:10.1016/j.watres.2012.07.005 |
| [5] |
ZHU L, SANTIAGO S B, XIAO H, et al. Electrochemical oxidation of fluoroquinolone antibiotics: Mechanism, residual antibacterial activity and toxicity change[J]. Water Research, 2016, 102: 52-62. DOI:10.1016/j.watres.2016.06.005 |
| [6] |
LIN H H, LIN Y C. Photocatalytic oxidation of 5-fluorouracil and cyclophosphamide via UV/TiO2 in an aqueous environment[J]. Water Research, 2014, 48: 559-568. DOI:10.1016/j.watres.2013.10.011 |
| [7] |
WOLS B A, HOFMAN C H M, HARMSEN D J H, et al. Degradation of 40 selected pharmaceuticals by UV/H2O2[J]. Water Research, 2013, 47(15): 5876-5888. DOI:10.1016/j.watres.2013.07.008 |
| [8] |
MICHAEL K I, IACOVOU M, FRONTISTIS Z, et al. Erythromycin oxidation and ERY-resistant Escherichia coli inactivation in urban wastewater by sulfate radical-based oxidation process under UV-C irradiation[J]. Water Research, 2015, 85: 346-358. DOI:10.1016/j.watres.2015.08.050 |
| [9] |
WANG Y, COUET M, GUTIERREZ L, et al. Impact of DOM source and character on the degradation of primidone by UV/chlorine: Reaction kinetics and disinfection by-product formation[J]. Water Research, 2020, 172: 115463. DOI:10.1016/j.watres.2019.115463 |
| [10] |
CAI W W, PENG T, ZHANG J N, et al. Degradation of climbazole by UV/chlorine process: Kinetics, transformation pathway and toxicity evaluation[J]. Chemosphere, 2019, 219: 243-249. DOI:10.1016/j.chemosphere.2018.12.023 |
| [11] |
周思琪, 李佳琦, 杜尔登, 等. UV/Cl工艺对三氯生的去除与降解机理研究[J]. 中国环境科学, 2019, 39(3): 1000-1008. ZHOU S Q, LI J Q, DU E D, et al. Removal and degradation mechanism of triclosan by the UV/chlorine process[J]. China Environmental Science, 2019, 39(3): 1000-1008. DOI:10.3969/j.issn.1000-6923.2019.03.013 |
| [12] |
GUO K H, WU Z H, SHANG C, et al. Radical chemistry and structural relationships of PPCP degradation by UV/chlorine treatment in simulated drinking water[J]. Environmental Science & Technology, 2017, 51(18): 10431-10439. |
| [13] |
BU L, ZHOU S, ZHU S, et al. Insight into carbamazepine degradation by UV/monochloramine: Reaction mechanism, oxidation products, and DBPs formation[J]. Water Research, 2018, 146: 288-297. DOI:10.1016/j.watres.2018.09.036 |
| [14] |
XIANG Y Y, FANG J Y, SHANG C. Kinetics and pathways of ibuprofen degradation by the UV/chlorine advanced oxidation process[J]. Water Research, 2016, 90: 301-308. DOI:10.1016/j.watres.2015.11.069 |
| [15] |
WU Z H, FANG J Y, XIANG Y Y, et al. Roles of reactive chlorine species in trimethoprim degradation in the UV/chlorine process: Kinetics and transformation pathways[J]. Water Research, 2016, 104: 272-282. DOI:10.1016/j.watres.2016.08.011 |
| [16] |
CAI W W, PENG T, YANG B, et al. Kinetics and mechanism of reactive radical mediated fluconazole degradation by the UV/chlorine process: Experimental and theoretical studies[J]. Chemical Engineering Journal, 2020, 402: 126224. DOI:10.1016/j.cej.2020.126224 |
| [17] |
BELGHIT A, MEROUANI S, HAMDAOUI O, et al. Influence of processing conditions on the synergism between UV irradiation and chlorine toward the degradation of refractory organic pollutants in UV/chlorine advanced oxidation system[J]. Science of the Total Environment, 2020, 736: 139623. DOI:10.1016/j.scitotenv.2020.139623 |
| [18] |
NIKRVESH B, SHOMALNASAB A, NAYYER A, et al. UV/Chlorine process for dye degradation in aqueous solution: Mechanism, affecting factors and toxicity evaluation for textile wastewater[J]. Journal of Environmental Chemical Engineering, 2020, 8(5): 104244. DOI:10.1016/j.jece.2020.104244 |
| [19] |
LEE J Y, LEE Y M, KIM T K, et al. Degradation of cyclophosphamide during UV/chlorine reaction: Kinetics, byproducts, and their toxicity[J]. Chemosphere, 2021, 268: 128817. DOI:10.1016/j.chemosphere.2020.128817 |
| [20] |
BOLZ U, HAGENMAIER H, KOMER W. Phenolic xenoestrogens in surface water, sediments, and sewage sludge from Baden-Württemberg, south-west Germany[J]. Environmental Pollution, 2001, 115(2): 291-301. DOI:10.1016/S0269-7491(01)00100-2 |
| [21] |
DAVOREN M, FOGARTYA M. Ecotoxicological evaluation of the biocidal agents sodium o-phenyl phenol, sodium o-benzyl-p- chlorophenol, and sodium p-tertiary amylphenol[J]. Ecotoxicology and Environmental Safety, 2005, 60(2): 203-212. DOI:10.1016/j.ecoenv.2004.04.004 |
| [22] |
WHO International Agency for Research on Cancer (IARC) List of carcinogens [EB/OL]. (2007-11-04) [2021-07-22]http://samr.cfda.gov.cn/WS01/CL1991/215896.html.
|
| [23] |
YE B, LI Y, CHEN Z, et al. Degradation of polyvinyl alcohol (PVA) by UV/chlorine oxidation: Radical roles, influencing factors, and degradation pathway[J]. Water Research, 2017, 124: 381-387. DOI:10.1016/j.watres.2017.05.059 |
| [24] |
PAN Y, LI X, FU K, et al. Degradation of metronidazole by UV/chlorine treatment: Efficiency, mechanism, pathways and DBPs formation[J]. Chemosphere, 2019, 224: 228-236. DOI:10.1016/j.chemosphere.2019.02.081 |
| [25] |
FANG J Y, FU Y, SHANG C. The roles of reactive species in micropollutant degradation in the UV/free chlorine system.[J]. Environmental Science & Technology, 2014, 48(3): 1859-1868. |
| [26] |
JENNINGS V L K, RAYNER B M H, BIRD D J. Assessing chemical toxicity with the bioluminescent photobacterium (vibrio fischeri): A comparison of three commercial systems[J]. Water Research, 2001, 35(14): 3448-3456. DOI:10.1016/S0043-1354(01)00067-7 |
| [27] |
KLANING U K, WOLFF T J. Laser flash photolysis of HClO, CIO-, HBrO, and BrO- in aqueous solution. Reactions of Cl- and Br-atoms[J]. Berichte der Bunsengesellschaft/Physical Chemistry Chemical Physics, 1985, 89(3): 243-245. DOI:10.1002/bbpc.19850890309 |
| [28] |
ANASRASIO C, MATTHEW B. A chemical probe technique for the determination of reactive halogen species in aqueous solution: Part 1 bromide solutions[J]. Atmos Atmospheric Chemistry and Physics, 2006, 6(9): 2423-2437. DOI:10.5194/acp-6-2423-2006 |
| [29] |
ZEHAVI D, RABANI J. Oxidation of aqueous bromide ions by hydroxyl radicals. Pulse radiolytic investigation[J]. Journal of Physical Chemistry, 1972, 76(3): 312-319. DOI:10.1021/j100647a006 |
| [30] |
BEITZ T, BECHMANN W, MITZNER R. Investigations of reactions of selected azaarenes with radicals in water. 2. Chlorine and bromine radicals[J]. Journal of Physical Chemistry A, 1998, 102(34): 6766-6771. DOI:10.1021/jp980655a |
| [31] |
NETA P, HUIE R E, ROSS A B. Rate constants for reactions of inorganic radicals in aqueous solution[J]. Journal of Physical and Chemical Reference Data, 1988, 17(3): 1027-1284. DOI:10.1063/1.555808 |
| [32] |
BUXTON G V, GREENSTOCK C L, HELMAN W P, et al. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O-) in aqueous solution[J]. Journal of Physical and Chemical Reference Data, 1988, 17(2): 513-886. DOI:10.1063/1.555805 |
| [33] |
XIONG R, LU Z, TANG Q, et al. UV-LED/chlorine degradation of propranolol in water: Degradation pathway and product toxicity[J]. Chemosphere, 2020, 248: 125957. DOI:10.1016/j.chemosphere.2020.125957 |
| [34] |
DENG J, WU G X, YUAN S J, et al. Ciprofloxacin degradation in UV/chlorine advanced oxidation process: Influencing factors, Mechanisms and degradation pathways[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2019, 371: 151-158. DOI:10.1016/j.jphotochem.2018.10.043 |
| [35] |
LEI Y, CHENG S, LUO N, et al. Rate constants and mechanisms of the reactions of Cl· and Cl2·- with trace organic contaminants[J]. Environmental Science & Technology, 2019, 53(19): 11170-11182. |
| [36] |
LIAN L, YAO B, HOU S, et al. Kinetic study of hydroxyl and sulfate radical-mediated oxidation of pharmaceuticals in wastewater effluents[J]. Environmental Science & Technology, 2017, 51(5): 2954-2962. |
| [37] |
GUO K H, WU Z H, YAN S W, et al. Comparison of the UV/chlorine and UV/H2O2 processes in the degradation of PPCPs in simulated drinking water and wastewater: Kinetics, radical mechanism and energy requirements[J]. Water Research, 2018, 147: 184-194. DOI:10.1016/j.watres.2018.08.048 |
| [38] |
DAVID A A, ROBERT E H, ERGEI L, et al. Standard electrode potentials involving radicals in aqueous solution: Inorganic radicals[J]. Bioinorganic Reaction Mechanisms, 2013, 9(1/2/3/4): 59-61. |
| [39] |
YANG T, MAI J, WU S, et al. UV/chlorine process for degradation of benzothiazole and benzotriazole in water: Efficiency, mechanism and toxicity evaluation[J]. Science of The Total Environment, 2021, 760(1): 144304. |
| [40] |
WANG W L, WU Q Y, HUANG N, et al. Synergistic effect between UV and chlorine (UV/chlorine) on the degradation of carbamazepine: Influence factors and radical species[J]. Water Research, 2016, 98: 190-198. DOI:10.1016/j.watres.2016.04.015 |
| [41] |
LIU Y, HE X, DUAN X, et al. Significant role of UV and carbonate radical on the degradation of oxytetracycline in UV-AOPs: Kinetics and mechanism[J]. Water Research, 2016, 95: 195-204. DOI:10.1016/j.watres.2016.03.011 |
| [42] |
LARSON R A, ZEPP R G. Reactivity of the carbonate radical with aniline derivatives[J]. Environmental Toxicology & Chemistry, 2010, 7(4): 265-274. |
| [43] |
MOORE J S, PHILLIPS G O, SOSNOWSKI A. Reaction of the carbonate radical anion with substituted phenols[J]. Proceedings of the Indian Academy of Sciences-Section A, 1984, 31(6): 603-605. |
| [44] |
KONG Q, LEI X, ZHANG X, et al. The role of chlorine oxide radical (ClO·) in the degradation of polychoro-1, 3-butadienes in UV/chlorine treatment: Kinetics and mechanisms[J]. Water Research, 2020, 183: 116056. DOI:10.1016/j.watres.2020.116056 |
| [45] |
YIN R, SHANG C. Removal of micropollutants in drinking water using UV-LED/chlorine advanced oxidation process followed by activated carbon adsorption[J]. Water Research, 2020, 185: 116297. DOI:10.1016/j.watres.2020.116297 |
| [46] |
ZHANG Y, ZHANG J, XIAO Y, et al. Kinetic and mechanistic investigation of azathioprine degradation in water by UV, UV/H2O2 and UV/persulfate[J]. Chemical Engineering Journal, 2016, 302: 526-534. DOI:10.1016/j.cej.2016.05.085 |
| [47] |
韩志涛, 杨少龙, 郑德康, 等. 紫外辐照强化NaClO溶液湿法脱硝的实验研究[J]. 科学技术与工程, 2016, 16(28): 134-138, 170. HAN Z T, YANG S L, ZHENG D K, et al. No removal from simulated flue gas by UV-irradiated sodium hypochlorite solution[J]. Science Technology and Engineering, 2016, 16(28): 134-138, 170. DOI:10.3969/j.issn.1671-1815.2016.28.023 |
| [48] |
李博强, 马晓雁, 李青松, 等. UV-LED/NaClO工艺对水中对乙酰氨基酚的降解[J]. 中国环境科学, 2019, 39(11): 4681-4688. LI B Q, MA X Y, LI Q S, et al. Degradation of acetaminophen in aqueous by UV-LED/NaClO process[J]. China Environmental Sciencece, 2019, 39(11): 4681-4688. DOI:10.3969/j.issn.1000-6923.2019.11.025 |
| [49] |
KIM T K, KIM T, CHA Y, et al. Energy-efficient erythromycin degradation using UV-LED (275 nm)/chlorine process: Radical contribution, transformation products, and toxicity evaluation[J]. Water Research, 2020, 185: 116159. DOI:10.1016/j.watres.2020.116159 |
| [50] |
KONG X, WU Z, REN Z, et al. Degradation of lipid regulators by the UV/chlorine process: Radical mechanisms, chlorine oxide radical (ClO·)- mediated transformation pathways and toxicity changes[J]. Water Research, 2018, 137: 242-250. DOI:10.1016/j.watres.2018.03.004 |
| [51] |
ESPLUGAS S, GIMENEZ J, CONTRERAS S, et al. Comparison of different advanced oxidation processes for phenol degradation[J]. Water Research, 2002, 36(4): 1034-1042. DOI:10.1016/S0043-1354(01)00301-3 |
| [52] |
GAO Z C, LIN Y L, XU B, et al. Evaluating iopamidol degradation performance and potential dual-wavelength synergy by UV-LED irradiation and UV-LED/chlorine treatment[J]. Chemical Engineering Journal, 2019, 360: 806-816. DOI:10.1016/j.cej.2018.12.022 |
| [53] |
WU Z G, GUO K H, FANG J Y, et al. Factors affecting the roles of reactive species in the degradation of micropollutants by the UV/chlorine process[J]. Water Research, 2017, 126: 351-360. DOI:10.1016/j.watres.2017.09.028 |




