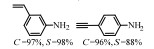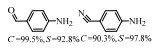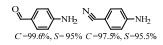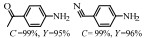2. 浙江大学衢州研究院, 浙江 衢州 324000
2. Institute of Zhejiang University-Quzhou, Quzhou 324000, China
苯胺类衍生物是一类重要的精细化工中间体,作为原料广泛应用于医药、农药、橡胶和染料等化学品的生产中[1-2]。工业上常通过硝基芳烃的还原制备苯胺类化合物,一般包括铁粉还原法,硫化碱还原法,电化学还原法,转移氢化法和催化加氢法等。其中,催化加氢法以氢气为还原剂,具有优异的经济性和绿色环保性,是工业上制备苯胺的常用手段。但是,当底物分子中存在其他敏感性基团时,催化加氢过程中极易发生过度加氢生成副产物[3-4],这不仅降低了反应收率,而且增加了后续的分离纯化难度。因此,如何实现硝基芳烃的高效选择性催化加氢已经成为研究的焦点。
根据底物分子上取代基类型的不同,硝基芳烃选择性催化加氢反应主要可分为2类:一是卤代硝基芳烃的选择性加氢;二是含有其他不饱和官能团的硝基芳烃的选择性加氢。对于前者而言,一方面硝基和卤素原子会在加氢活性位点发生竞争性吸附,另一方面还原产物中的氨基通过诱导电子转移增强卤素原子上的电子云密度,从而削弱碳-卤键强度,使卤素在反应过程中极易脱落。一般来说,随着取代基F、Cl、Br、I的电负性依次减弱,碳-卤键也越容易断裂[5]。除卤素外,当底物中含有碳-碳不饱和键(如─C═C、─C≡C)或碳-杂原子不饱和键(如─C≡N、─C═O)时,加氢极易发生在该类不饱和键上,其中含有─C═C和─C≡C的硝基芳烃是具有挑战性的底物。一些复杂的底物分子同时含有多个敏感基团,进一步增大了选择性还原硝基的难度。为解决该类底物低选择性转化的问题,各类高效选择性加氢催化剂被广泛报道,本综述较系统地介绍了近年来硝基芳烃选择性加氢催化剂(非均相)的研究进展。
2 贵金属催化剂钯(Pb)、铂(Pt)、钌(Ru)等贵金属催化剂具有较高的加氢活性,已经广泛用于硝基芳烃催化加氢反应中。然而,简单的负载型贵金属催化体系普遍存在选择性较低的问题,限制了其在硝基芳烃还原中的进一步应用。针对贵金属催化体系选择性低的难题,近年来研究者们提出了多种调节及改性策略,主要包括调变载体种类及性质、双金属协同催化、单原子催化、金属界面效应和空间限域效应等等。
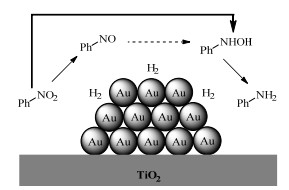
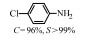
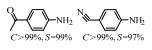
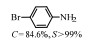
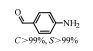
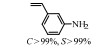
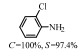
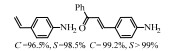
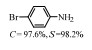
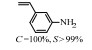
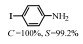
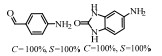
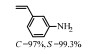
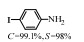
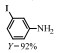
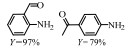
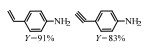
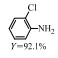
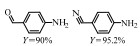
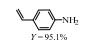
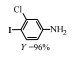
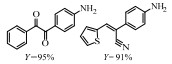
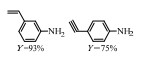
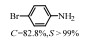
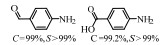
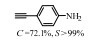
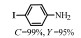
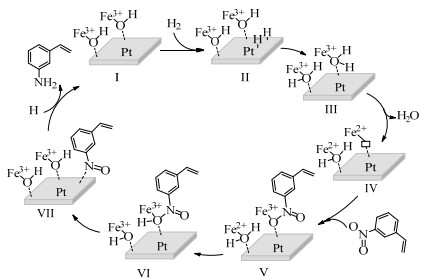
2.1 调变载体种类及性质对于负载型催化剂而言,载体的种类及性质对催化活性与选择性具有关键影响。金属氧化物是最常用的载体,金属/氧化物选择性催化加氢体系已有大量报道,比如Pt/Fe2O3[6]、Au/SiO2[5]、Au/Fe2O3[7]、Pt/ZnO[8-9],Pd/ZnO[10]、Au/Al2O3[11]、Ag/Al2O3[12]、Pd/CeO2[13]等等。其中,Boronat等[14]和Corma等[7,15-16]的研究表明,还原性载体TiO2可以通过金属-载体的强相互作用(strong metal-support interaction,SMSI)及对硝基的优先吸附,有效提升催化活性与选择性。当底物上有乙烯基、醛基、氰基和碘等取代基时,Au/TiO2、Pt/TiO2、Ru/TiO2和Ni/TiO2在保持高转化率的同时能够实现85%~100% 的选择性(见图1)。
基于Corma等的工作,一系列以TiO2为载体的贵金属催化剂被开发并应用到了硝基芳烃选择性加氢反应中,如Au/TiO2[17]、Au/TiO2/UVM-7[18]、Au-MTA[19]、Pt/TiO2[20]、Pd/TiO2[10]、PtTW[21]等等。2019年,Macino等[22]指出Pt/TiO2界面处的外围位点可能是硝基芳烃选择性加氢的活性位点。在还原的条件下,Pt负载质量分数为0.2% 和0.5% 的催化剂会产生SMSI效应,使得TiOx覆盖在活性Pt位点表面,从而降低了催化活性与选择性,可以通过对催化剂的预煅烧防止这一现象发生。最近,Zhang等[23]进一步研究了不同晶型TiO2对催化活性的影响,发现Ru/TiO2(金红石)主要促进偶联过程,而Ru/TiO2(锐钛矿)则可以高选择性得到各类取代芳胺(转化率和选择性均大于99.9%)。
对氧化物进行改性,可以进一步提升其与金属或底物的相互作用,已报道的体系如PtTW[21]、Fe3O4-NH2-Pd[24]、Fe3O4@PPy-Pt[25]等等。2017年,Tamura等[26]用金属氧化物(MoOx、WOx和ReOx)对Ru/SiO2进行改性,发现Ru-MoOx/SiO2(Mo、Ru物质的量比为1:2)对各类还原性官能团取代的芳硝基底物都具有高活性与选择性(均大于90%)。研究表明,Ru与MoOx的界面处会形成活性位点,在该位点上,MoOx对底物有强吸附作用,而Ru上则形成了活性氢化物,从而显著提升了活性和选择性。2018年,Wang等[27]在TiO2载体上引入单位点的Sn,Sn─O─Ti键有利于TiO2上氧空位的形成,促进硝基脱氧生成亚硝基,而贵金属则为亚硝基加氢提供了活性位点。以Sn-TiO2为载体,Au、Pt、Ru、Ni对各类硝基底物的催化活性和选择性均得到显著提升。
除氧化物载体之外,一些表面含有杂原子掺杂的炭材料也可以充当硝基的吸附位点,相应的体系如Pd-Ph2S/C[28]、Au/C(En)[29]、Ru/CN[30]、Ir/MWCNTs[31]、Pt/N-CMK-3[32]、Ru/CNTs[33]、Pd-P-C[34]等等。2018年,Wu等[35]通过硝酸和磷掺杂对活性炭进行了前处理,制备了一种可转换产物选择性的Pt/C催化剂。结果表明,经过硝酸处理后,活性炭表面的酸性含氧官能团数量显著增加,而炭载体表面官能团和杂原子磷对催化过程产生关键影响(见图2)。在低温还原的催化剂中,靠近Pt纳米颗粒的酸性基团通过氢键与底物的硝基相互作用,使乙烯基更靠近活性金属中心,选择性加氢生成1-乙基-3-硝基苯(转化率95%,选择性93%);而在催化剂被高温还原的过程中,P与Pt通过相互作用形成Pt-POx复合物,这种复合物更倾向于吸引极性硝基,从而选择性生成对氨基苯乙烯(转化率91%,选择性96%)。
|
图 2 Pt/ACH-150和Pt/ACH-450催化剂上3-硝基苯乙烯选择性加氢示意图[35] Fig.2 Schematic illustration of selective hydrogenation of 3-nitrostyrene on Pt/ACH-150 and Pt/ACH-450 catalysts[35] |
构建双金属催化剂是调变活性与选择性的有效策略。通常来说,一种金属组分(往往是贵金属)具有较高的催化活性,另一种则主要起到调节结构或电性的作用,二者协同提升活性与选择性。近年来报道的双金属催化体系有PtSn/H-MoOx[36]、Ru3Ni1[37]、Ni-Au/C[38]、NHC@AuPd/TiO2[39]、PdCo@SiO2[40]、RhIn/SiO2[41]、Cu/C-Pt[42]、Co/Pt/PAC[43]、Pt-Zn/SiO2[44]等等。2017年,Mao等[45]将一定量的Co原子掺入Ru中使其产生晶格应变,并通过高分辨透射电镜和X射线吸收精细结构研究证实Ru晶格的压缩现象,Ru─Ru键的收缩程度随Co含量升高而增大。实验结果表明,当Ru存在3% 的晶格应变时,其对4-硝基苯乙烯的加氢选择性从66% 提高到99%。密度泛函理论(density functional theory,DFT)结果表明,优化的横向压缩应变在阻碍乙烯基的氢化的同时促进了硝基的氢化。
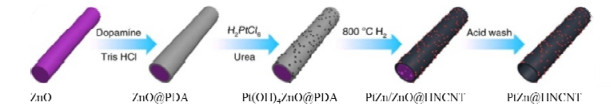
同年,Pei等[46]合成了一系列金属间化合物PtM-和Pt3M-型催化剂(M = Sn, Pb, Zn)。相较于Pt和Pt3M-型,PtM-型金属间化合物在3-硝基苯乙烯催化加氢中展现了优异的选择性(> 99%),其中PtSn@mSiO2的转化率为99%。研究表明,PtSn的表面结构不仅改变了加氢途径,而且促进了硝基的优先吸附。2019年,Han等[47]通过牺牲模板法在氮掺杂碳纳米管上制备了一种PtZn金属间化合物(见图3),在该催化剂中,Pt原子比Zn原子具有更高的电子密度,Zn原子促进了硝基的吸附与氢原子的扩散。该催化剂在对硝基苯乙炔选择性加氢中的转化率与选择性均超过99%,优于Pt单原子催化剂。
|
图 3 中空氮掺杂碳纳米管负载PtZn金属间化合物纳米颗粒[47] Fig.3 PtZn intermetallic nanoparticles supported on hollow nitrogen-doped carbon nanotubes[47] |
单原子催化剂(single atom catalyst,SAC)是Qiao等[48]于2011年提出的概念,他们利用FeOx表面的缺陷锚定Pt原子,发现当Pt负载质量分数为0.08%,且还原温度为200 ℃时,Pt表现出类似孤立原子的结构,通过同步辐射证明了质量分数为0.08% 的Pt/FeOx中不存在强Pt─Pt金属键。2014年,Wei等[3]将该类催化剂首次应用到硝基芳烃的选择性加氢反应中,在40 ℃,0.3 MPa H2下对于各种官能化的底物都具有极高的活性(> 90%)和选择性(> 98%),转换频率(turnover frequency,TOF)高达1 500 h−1。作者将优异的催化性能归因于孤立的活性位点,SMSI效应以及硝基的优先吸附。2017年,Wei等[49]进一步提高了Pt的负载量,并探讨了碱金属(Li+,Na+,K+等)对质量分数为2.16% 的Pt/FeOx催化性能的影响。他们发现,一定量Na+的加入可以在保持3-硝基苯乙烯高转化率的情况下,将选择性从66.4% 提高到97.4%。研究表明,Na+与Pt/FeOx催化剂通过相互作用形成了Pt-O-Na-O-Fe物种,这不仅防止了Pt原子的聚集,而且促进了硝基基团的优先吸附。
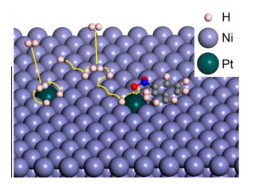
基于Shi等[50]的开创性工作,一系列单原子催化剂被开发并应用于硝基芳烃的选择性催化加氢中。2018年,Peng等[51]在Ni纳米晶体表面掺入Pt单原子,构建了底物均匀吸附的催化剂构型(见图4)。在温和条件下,该催化剂对硝基苯乙烯的TOF值可达1 800 h−1,选择性大于99%,且对于各种官能团取代的底物都表现出了适用性。研究表明,H2会在Pt和Ni原子上自发解离,生成的H原子在催化剂表面极易扩散,为加氢过程提供了充足的氢。此外,Pt单原子和周围的Ni原子协同诱导了3-硝基苯乙烯的吸附构型,促进了硝基的优先活化。2019年,Lin等[52]制备了一种原子分散的Pt/α-MoC催化剂,该催化剂即使在5 000×10−6 CO的存在下,也能实现对3-硝基苯乙烯催化加氢100% 的转化率和99.9% 的选择性。研究表明,Pt原子与α-MoC之间的相互作用削弱了CO在Pt上的吸附,独特的界面结构降低了硝基的加氢能垒。此外,水的加入不仅促进了加氢过程,还增强了抗CO毒化性能。
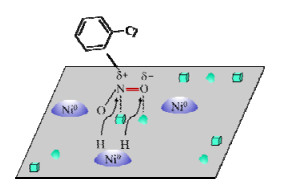
2.4 其他改性策略除了上述策略,近年来也报道了一些新型的催化剂改性方法。比如,Chen等[53]提出可以通过界面效应提高催化加氢选择性。2020年[54],他们发现在Pt纳米粒子(nanoparticles,NPs)上沉积Fe(OH)x可以有效促进硝基的选择性氢化,选择性达到90% 以上。作者指出,选择性的提高得益于Fe(III)-OH-Pt的界面,H2容易在暴露的Pt原子上分解为H原子,然后迁移到界面与OH反应生成H2O和Fe2+,H2O的释放导致Fe2+附近形成空位,Fe2+的亲氧性使其可以选择性捕获并还原硝基(见图5)。此外,利用分子筛的空间限域效应促进硝基的选择性吸附也是一种新策略[55-56]。如2017年,Zhang等[57]采用晶种定向合成方法将Pd NPs包封固定在Beta沸石中,得到的Pd@Beta催化剂对4-氯苯胺和4-氨基苯甲醛具有超过99% 的选择性,而负载型催化剂(Pd/C,Pd/TiO2,Pd/Al2O3,Pd/SiO2)对4-氯苯胺的选择性范围仅为70.9%~89.6%。研究表明,沸石微孔改变了底物分子与Pd位点的吸附空间排列,使4-氯硝基苯通过硝基与Pd位点作用。利用该策略制备得到的Pd@MOR(MOR为丝光沸石),Ru@Beta和Pt@Beta均展现了优异的活性与选择性。
|
图 5 硝基苯在Fe(OH)x/Pt表面催化脱氧机理[54] Fig.5 Mechanism of catalytic deoxygenation of nitrobenzene on Fe(OH)x/Pt surface[54] |
近年来,储量丰富、价格低廉、环境友好的非贵金属如Fe、Co、Ni、Cu作为硝基芳烃选择性加氢催化剂也取得了一定进展[58]。其中,Co基和Ni基催化剂得到了广泛研究,Fe基和Cu基催化剂也有少量报道。
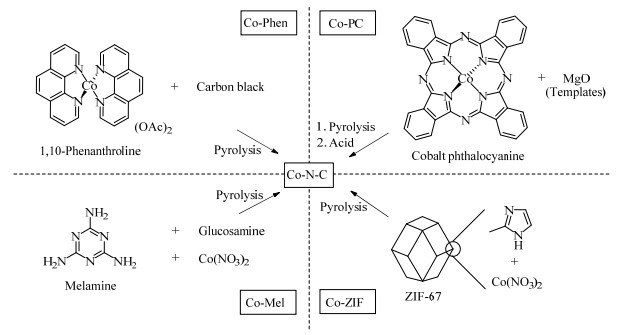
3.1 钴基催化剂在非贵金属选择性加氢催化剂中,以钴基催化剂的研究最为广泛。2013年,Westerhaus等[59]报道了一种钴-含氮配合物煅烧策略,研究发现,不同有机配体制备的催化剂在加氢活性上有显著差异,其中由1,10-菲罗啉制备而成的Co-N-C复合催化剂具有最高的催化活性(见图6),对各类官能团取代的硝基芳烃都表现出优异的活性与选择性,该策略也被用于制备镍基[60]与铁基催化剂[61]。2017年,Formenti等[62]更换配体为α-二亚胺类分子,采用同样的方法制备了新型Co催化剂,但催化活性相对1,10-菲罗啉并没有明显的提升,通过X射线光电子能谱(X-ray photoelectron spectroscopy,XPS)和动力学实验推测未配位的吡啶二氮原子在催化活性中起着关键作用。同年,Sahoo等[63]又使用壳聚糖作为载体,利用壳聚糖上的氨基和羟基与钴配位,制备了类似的材料,但催化能力较前2种催化剂反而有所下降。
|
图 6 Co-菲罗啉络合物高温热解制备Co-N-C复合催化剂[59] Fig.6 Co-N-C composite catalysts prepared by high temperature pyrolysis of Co-phenanthroline complex[59] |
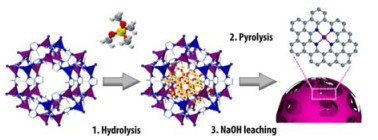
基于上述工作,大量Co/NC型催化材料被陆续报道[64-68],其中金属有机框架材料(metal organic frameworks,MOFs)因其高度有序的空间结构和可调的配体,被认为是理想的牺牲模板[69-73]。2020年,Wang等[74]以Zn/Co双金属ZIF骨架为前体,采用高温热解蒸发Zn原子的策略制备得到了负载在氮掺杂炭上的Co单原子催化剂(Co SAs/NC)。该催化剂中Co的质量分数为1.33%,且1个Co原子与4个氮原子配位,以单原子的形式存在。Co SACs/NC对各种取代的硝基底物都展现了优异的活性与选择性(均大于97%)。此外,作者对反应的溶剂效应进行了研究,发现在溶剂乙醇中加入适量水有利于硝基苯的吸附和苯胺的解吸,从而提升反应活性与选择性。除了单一MOFs热解外,还可以结合SiO2模板法进一步调节催化剂结构[75]。2018年,Sun等[76]通过将原硅酸四甲酯(TMOS)在Zn/Co双金属ZIF中水解,合成了具有介孔结构和原子分散位点的Co@mesoNC催化剂(见图7)。ZIF前体结构中大量Zn和N的存在以及孔隙中SiO2可以有效阻止Co原子聚集。该催化剂在还原性官能团取代的硝基底物催化加氢中展现出优异的选择性(>93%)。
通常,热解得到的Co/NC催化剂在组成上较为复杂,具有多种可能存在的加氢活性位点,比如金属/氧化物纳米颗粒、Co-Nx物种或载体表面掺杂的氮氧基团,而活性物种的归属问题长期也存在争议。2021年,Li等[77]系统考察4种文献报道的Co/NC催化剂(见图8),其在3-硝基苯乙烯选择性加氢反应中均表现出优异的活性与选择性。溶剂效应表明,4种催化剂均在质子型溶剂中具有较高的活性,说明活性中心与质子型溶剂间可能存在由单原子Co-Nx结构(类似于均相催化剂)所介导的质子穿梭效应。此外,研究者还通过毒化实验、酸洗实验和催化剂再生实验揭示了单原子物种Co-Nx物种是反应的活性位点。
除了氮掺杂之外,其他杂原子掺杂的Co基催化剂也有报道[70-71,78]。2020年,Zhang等[79]利用牛血清白蛋白上的S原子固定Zn2+和Co2+,形成了蛋白质-金属离子网络,热解过程中金属离子被还原,通过进一步的酸处理形成了Co、N掺杂的多孔炭结构。表征结果表明,Zn2+的引入有利于Co分散和多孔结构的形成,酸处理可以去除大的Co纳米颗粒,从而促进活性位点的暴露。此外,催化剂中部分Co以CoyZnS的形式存在,另一部分则形成了Co-Nx。该催化剂不仅对于各类硝基芳烃底物有着优异的催化活性(转化率100%,选择性均大于98%),还可以抵抗CO或H2S的毒化。
3.2 镍基催化剂雷尼镍是常见的工业镍基催化剂,常用于有机化合物的加氢还原,但是与贵金属一样,它也具有较低的催化加氢选择性。通过对雷尼镍的改性可以有效提高其选择性,如2012年,Lu等[80]发现双氰胺改性过的雷尼镍能将邻氯硝基苯的反应时间缩短3/4以上,选择性接近100%。作者通过实验指出,雷尼镍与双氰胺分子中的氮原子之间存在很强的相互作用,可以覆盖电子缺陷的Lewis酸位点Ni-H,从而切断Cl…H-Ni中间态的形成,使脱氯受到抑制。2015年,Liu等[81]研究了金属氟化物对雷尼镍的影响,发现不同的金属氟化物都能提升反应活性与选择性,其中CaF2 > NaF ≈ KF > MgF2 > 无氟化物。作者推测,添加的氟化物会沉积在催化剂表面,从而改变了雷尼镍的电子性质,但文中并没有给出相应的证明。
开发新型镍基催化剂是另一种可行的思路[82-85],比如通过对载体的改性提升催化活性与选择性[86]。2016年,Li等[87]制备出具有丰富表面缺陷的镍纳米催化剂层状双金属氢氧化物NiTi-LDH(见图9)。研究表明,金属与载体之间具有强相互作用,且载体上形成了大量的氧空位和Ti3+物种。作者推测,表面氧空位会作为电子给体吸引氮原子,引发N─O键的活化,而Ti3+阳离子可能会与硝基中带负电的氧原子结合,从而定向诱导硝基的吸附。反应动力学也证实表面缺陷对邻氯硝基苯的氢化起到促进作用。2017年,Ren等[88]先用硝酸处理活性炭,再通过负载质量分数为5% 镍还原得到一种改性催化剂Ni/ACox。载体表面生成的氧基团有助于稳定Ni NPs,使之具有更均匀的分布和更小的尺寸((5.5±0.8) nm和(8.3±2.3) nm)。研究表明,Ni/ACox能在非常温和的条件下(40 ℃,0.3 MPa H2),对3-硝基苯乙烯的氢化反应实现98% 的转化率和97% 的选择性,此外,该催化剂对其他还原性基团也有很好的耐受性。最近,Huang等[89]制备的TiO2@OAC载体结合了NiTi-LDH和ACox 2种载体的优势,在负载金属镍后,对氯代硝基苯氢化具有优异的催化性能。
|
图 9 NiTi-x可能的加氢机理示意图[87] Fig.9 Possible hydrogenation mechanism on the as-formed NiTi-x catalysts (cube denotes oxygen vacancies, and green sphere denotes Ti3+ defects)[87] |
与Co/NC结构相似,以氮掺杂炭为载体的镍催化剂也展现了出色的催化性能[90-92]。2016年,Hahn等[84]报道了一种复合材料Ni@SiCN,以聚苯乙烯作为软模板,引进氮配位的镍络合物和聚硅氮烷,再通过交联、热解、模板脱除和还原的步骤获得介孔结构的催化剂。研究表明,Ni NPs分布均匀,平均粒径为5.5 nm。该催化剂对各种取代基都具有优异的耐受性。2020年,Advani等[93]以生物质壳聚糖为前体,通过浸渍-炭化法合成了一种Ni NPs负载的氮掺杂炭纳米管Ni@NCNT,Ni NPs均匀分布,尺寸在10~15 nm。该催化剂在0.5 MPa H2和50 ℃下成功实现各类硝基芳烃的高效选择性加氢。作者将催化剂的高活性和稳定性归因于镍和氮掺杂炭之间形成的异质结,具有较高费米能级的镍能自发地将电子提供给载体,从而在金属和载体的界面上建立一个负责选择性氢化的电荷区,促进了加氢的进行。
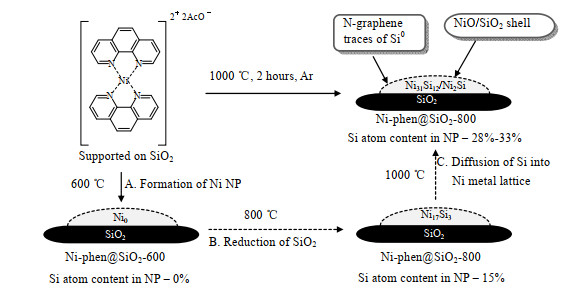
2018年,Ryabchuk等[94]以1,10-菲罗啉为配体制备一系列负载于无机载体上的氮掺杂镍基纳米催化剂,有趣的是,当以SiO2为载体时,在1 000 ℃下热解后会形成金属间硅化镍材料(见图10)。实验结果表明,热解温度对于硅化镍的形成至关重要,在800 ℃下,SiO2会发生还原,形成含量较低的Ni17Si3相;当温度升至1 000 ℃时,表面的硅原子进一步分布到镍晶格中,得到Ni31Si12/Ni2Si的混合物。将材料暴露于空气后,Ni-Si纳米颗粒的表面被氧化,形成NiO/SiO2壳。该催化剂具有很高的活性和选择性,能在温和条件下(60 ℃,1 MPa H2)实现各类硝基芳烃的高效选择性转化。
3.3 其他非贵金属催化剂除Co、Ni之外,铁基[95-98]、铜基及双金属复合催化剂也有报道。Jagadeesh等[61,99]报道了以H2为还原剂的铁基非均相催化体系,他们通过热解铁/菲罗啉配合物得到了一种具有氮掺杂石墨烯层包覆的活性Fe2O3 NPs。对于还原性官能团取代的硝基芳烃,该催化剂能以大于88% 的收率获得相应产物。2016年,Shi等[100]通过可再生生物质(葡萄糖、木糖醇和蔗糖)与三聚氰胺的直接热解,合成了嵌在碳纳米管中的Fe3C,且纳米颗粒被氮掺杂石墨烯层包裹。该催化剂在40 ℃下能实现各类卤素取代的硝基芳烃的高效选择性加氢。
Fe2P和FeS2[101-102]也被证明是有效的加氢活性位点。2018年,Zhu等[103]以Fe-MIL-88B-NH2为前体,进一步反应形成含P、S、N元素的聚合物外壳,最后一同热解制备了掺杂在碳基体中的磷化铁纳米材料。该Fe2P@C催化剂能高选择性地催化还原各种官能团取代的硝基芳烃,其活性是商用Fe2P粉末的5倍。2019年,Duan等[104]在N、S掺杂的多孔炭上负载FeS2纳米颗粒,得到一种具有高比表面积,高孔隙体积和分级孔道的新型催化剂FeS2/NSC。该催化剂能实现各种取代的芳硝基底物选择性加氢,催化性能优于其他文献报道的FeS2和FeOx类催化剂。实验和DFT计算表明,FeS2 NPs与氮和硫掺杂的载体具有较强的相互作用,电负性较强的N原子和S原子使FeS2 NPs带有正电,有利于对硝基的优先吸附。
有关铜基催化剂在H2气氛下还原硝基芳烃的报道相对较少[105],2016年,Kour等[106]报道了一种铜纳米颗粒修饰的氮掺杂炭纳米管。与前文的一些工作相似,作者用硫酸改性炭纳米管,使之表面产生含氧官能团,以碳酸胍作为掺杂用的氮源。在进行硝基芳烃的还原实验时,发现当在溶剂中加入乙酸,可以有效提高转化率(95%),且对于还原性基团取代的底物具有高度选择性。
此外,多种非贵金属复合形成的催化剂有时具有协同效应,展现优于单一金属的催化活性[107-108]。如2017年,Liu等[109]制备了具有薄碳涂层保护的Co@C、Ni@C、CoNi@C 3种催化剂。通过XPS等表征发现,Co@C表面由于空气的氧化作用覆盖着CoOx物种,而Ni的引入可以对金属Co起到稳定作用,同时增强了H2的解离作用。在对3-硝基苯乙烯的还原实验中,就催化活性而言,Ni@C > Co-Ni@C > Co@C,但是Ni@C催化剂的选择性仅为80%。相比之下,Co-Ni@C在保持一定的加氢活性的同时也显示了高选择性(>97%)。
4 无金属炭催化剂相较于金属催化剂,无金属炭材料性质稳定、价格低廉和绿色环保,具有不可比拟的优势。早在1985年,Han等[110]以石墨烯为催化剂,水合肼为还原剂,实现了硝基芳烃的还原。但受限于炭材料自身的催化活性较低,对H2和硝基的吸附较弱,且H2在其表面无法解离,一直以来,使用该类材料促进催化加氢过程的作用非常有限[111-112]。
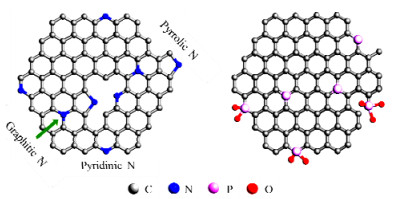
为了提升炭材料的氢化活性,在碳基质中掺杂无金属杂原子(如N、P、S、B等)是一种常见的改性手段(见图11)。掺杂的杂原子既可能充当路易斯酸或碱中心,又会改变炭材料的物理化学性质和氧化还原特性,比如引起电荷离域现象,使得炭材料呈现出类金属结构,有助于提升对H2的活化能力。此外,由于掺杂物和碳原子之间的配位键长和原子大小均不相同,杂原子周围通常会产生新的缺陷,缺陷位置的局部电荷富集,可以将更多的载流子转移到吸附的H2和硝基上,从而促进加氢过程的发生[113]。在已报道的材料中,石墨烯、富勒烯、Lewis酸-碱对固定炭材料、晶格缺陷碳和改性碳纳米管等材料都被尝试用于H2活化,与之相关的催化机理也纷纷被提出[114-115]。
2017年,Gao等[116]利用理论计算建立了4种模型,用于评估了磷掺杂和晶格缺陷对碳电子结构的影响。计算结果表明,电子会倾向于聚集在磷掺杂物和缺陷附近的碳原子上,电子离域使能带结构呈现类金属状态,其中,PV-C的费米能级像金属一样完全位于导带内。同时,也通过计算证实了磷掺杂及其引起的缺陷能有效激活H2分子和硝基。研究者基于计算结果合成了PV-C,发现其晶格缺陷浓度(ID/IG)与P掺杂浓度呈线性关系。在硝基芳烃的选择性加氢实验中,PV-C在2 MPa H2,120 ℃下,能够以优异的收率和选择性(>90%)还原多种芳硝基底物(包含不饱和官能团取代的底物)。值得注意的是,反应的TOF值也线性依赖于磷掺杂浓度和ID/IG。
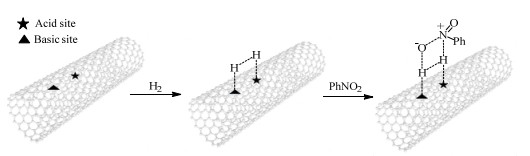
研究表明,对碳纳米管进行杂原子掺杂可以提升其氢化能力。如2020年,Chen等[112]对碳纳米管分别进行磷元素和氮元素掺杂,制备得到了P-CNT和N-CNT。与原始CNT相比,P-CNT和N-CNT都具有更大的比表面积和更多的晶格缺陷,且通过加入K2CO3添加剂可以进一步增加其表面缺陷程度。结果显示,P-CNT900具有最优异的催化性能,不仅能够实现相对温和条件下硝基苯的高效还原,而且对于含有敏感基团的硝基底物,也显示出优异的转化率与选择性。此外,该催化剂在循环8次后也没有明显的活性和选择性损失。文章指出掺杂到CNT基体中的磷原子可能会诱导表面电荷定位,促进CNT表面独立的受阻路易斯酸-碱对(frustrated Lewis pairs,FLP)的生成。这些FLP在反应过程中充当活性位点,通过H2极化和分裂,同时形成H−型和H+型位点来促进H2活化(见图12)。
|
图 12 以FLP为催化位点的P-CNT900加氢机理[112] Fig.12 Proposed mechanism of hydrogenation on P-CNT900 with FLP as catalytic active sites [112] |
在无金属炭催化剂的研究中,也有学者提出了质疑:在炭材料的合成过程中,尽管进行了酸或碱的处理,但是仍然不可避免地会有痕量金属残留,一些研究工作忽略了残留金属的影响,对催化中心的判断起到了误导作用[117-118]。典型的例子是,2009年,Li等[119]报道称利用富勒烯成功活化了分子氢。在紫外光辐射下(300 W高压Hg灯或350 W Xe灯),C60或C70的混合催化剂在室温和常压H2下就能实现硝基芳烃的高效催化加氢。在黑暗条件下,加氢过程在5 MPa H2,160 ℃的条件下也能顺利进行。但是次年,Pacosova等[120]就驳斥了这一发现,他们指出,在C60的合成过程中会使用镍,可能是残留的镍发挥了催化作用。对照实验表明,用镍还原法制备得到的C60会吸附溶液中的镍,而当用钠代替镍合成的C60是没有催化活性的。
总体来说,目前报道的无金属炭材料催化加氢体系普遍存在催化剂用量高、反应选择性低、反应条件苛刻、反应时间长等问题,且催化机理并不明晰。虽然无金属炭材料仍存在许多需要解决的问题,距离工业化尚远,但不可否认的是,它仍然是新型加氢催化剂研发的潜在方向。
5 催化剂的底物适用类型硝基芳烃分子中取代基的类型决定了其选择性加氢的难易程度,不同种类加氢催化剂往往具有不同的底物适用性。将前文重点介绍的各类代表性催化剂的底物适用类型统计于表1中,结果表明,经过催化剂的设计与改性,无论是贵金属催化剂、非贵金属催化剂还是无金属炭催化剂,都可能在优化条件下实现各类硝基芳烃的高效选择性加氢。
|
|
表 1 部分催化剂对不同类型硝基芳烃的选择性加氢性能 Table 1 Selective hydrogenation performance of catalysts for different types of nitroarenes |
综上所述,针对硝基芳烃选择性催化加氢反应已经开发了多种贵金属、非贵金属、无金属催化剂类型,且具有广泛的底物适用性。贵金属催化剂设计的难点在于控制选择性,可以通过调节金属相的组成和分散度、载体的表面性质与空间结构等手段改变催化剂与氢气或底物的作用方式,从而实现对硝基的优先活化。非贵金属催化剂的设计重点在于提高加氢活性,利用杂原子(N、S、P等)掺杂调变金属的电子性质与稳定性是目前最广泛使用的策略,但是大多数非贵金属催化剂仍存在反应条件较为苛刻的问题,如何实现温和条件下的高效加氢仍是目前的一大挑战。无金属炭催化剂廉价绿色,但其发展仍处于初步阶段,无论是反应活性还是催化机理均有较大的探索空间。总体来说,目前所报道的大多数催化剂虽然已经实现了较高的选择性催化加氢活性,但是其合成及应用大多停留在实验室阶段,且有的催化剂使用的前体昂贵,制备方法繁琐,稳定性较差,不适于进一步的工业应用,如何制备一种适用于工业生产的高效、高选择性、绿色、经济的催化剂仍是未来的研究方向。
| [1] |
DOWNING R S, KUNKELER P J, VANBEKKUM H. Catalytic syntheses of aromatic amines[J]. Catalysis Today, 1997, 37(2): 121-136. DOI:10.1016/S0920-5861(97)00005-9 |
| [2] |
BLASER H U, MALAN C, PUGIN B, et al. Selective hydrogenation for fine chemicals: Recent trends and new developments[J]. Advanced Synthesis & Catalysis, 2003, 345(1/2): 103-151. |
| [3] |
WEI H, LIU X, WANG A, et al. FeOx-supported platinum single-atom and pseudo-single-atom catalysts for chemoselective hydrogenation of functionalized nitroarenes[J]. Nature Communications, 2014, 5: 5634. DOI:10.1038/ncomms6634 |
| [4] |
SONG J, HUANG Z F, PAN L, et al. Review on selective hydrogenation of nitroarene by catalytic, photocatalytic and electrocatalytic reactions[J]. Applied Catalysis B-Environmental, 2018, 227: 386-408. DOI:10.1016/j.apcatb.2018.01.052 |
| [5] |
FORMENTI D, FERRETTI F, SCHARNAGL F K, et al. Reduction of nitro compounds using 3d-non-noble metal catalysts[J]. Chemical Reviews, 2019, 119(4): 2611-2680. DOI:10.1021/acs.chemrev.8b00547 |
| [6] |
EVANGELISTI C, ARONICA L A, BOTAVINA M, et al. Chemoselective hydrogenation of halonitroaromatics over gamma-Fe2O3-supported platinum nanoparticles: The role of the support on their catalytic activity and selectivity[J]. Journal of Molecular Catalysis A-Chemical, 2013, 366: 288-293. DOI:10.1016/j.molcata.2012.10.007 |
| [7] |
CORMA A, SERNA P. Chemoselective hydrogenation of nitro compounds with supported gold catalysts[J]. Science, 2006, 313(5785): 332-334. DOI:10.1126/science.1128383 |
| [8] |
BERGUERAND C, YARULIN A, CARDENAS-LIZANA F, et al. Chemoselective liquid phase hydrogenation of 3-nitrostyrene over Pt nanoparticles: Synergy with ZnO support[J]. Industrial & Engineering Chemistry Research, 2015, 54(35): 8659-8669. |
| [9] |
GAO T T, SHI W, ZHANG Y, et al. Finely controlled platinum nanoparticles over ZnO nanorods for selective hydrogenation of 3-nitrostyrene to 3-vinylaniline[J]. Chemistry-A European Journal, 2020, 26(41): 8990-8996. DOI:10.1002/chem.202001329 |
| [10] |
SUPRIYA P, SRINIVAS B T V, CHOWDESWARI K, et al. Biomimetic synthesis of gum acacia mediated Pd-ZnO and Pd-TiO2-promising nanocatalysts for selective hydrogenation of nitroarenes[J]. Materials Chemistry and Physics, 2018, 204: 27-36. DOI:10.1016/j.matchemphys.2017.10.026 |
| [11] |
SHIMIZU K I, MIYAMOTO Y, KAWASAKI T, et al. Chemoselective hydrogenation of nitroaromatics by supported gold catalysts: Mechanistic reasons of size- and support-dependent activity and selectivity[J]. Journal of Physical Chemistry C, 2009, 113(41): 17803-17810. DOI:10.1021/jp906044t |
| [12] |
SHIMIZU K I, MIYAMOTO Y, SATSUMA A. Size- and support-dependent silver cluster catalysis for chemoselective hydrogenation of nitroaromatics[J]. Journal of Catalysis, 2010, 270(1): 86-94. DOI:10.1016/j.jcat.2009.12.009 |
| [13] |
ZHANG S, CHANG C R, HUANG Z Q, et al. High catalytic activity and chemoselectivity of sub-nanometric Pd clusters on porous nanorods of CeO2 for hydrogenation of nitroarenes[J]. Journal of the American Chemical Society, 2016, 138(8): 2629-2637. DOI:10.1021/jacs.5b11413 |
| [14] |
BORONAT M, CONCEPCION P, CORMA A, et al. A molecular mechanism for the chemoselective hydrogenation of substituted nitroaromatics with nanoparticles of gold on TiO2 catalysts: A cooperative effect between gold and the support[J]. Journal of the American Chemical Society, 2007, 129(51): 16230-16237. DOI:10.1021/ja076721g |
| [15] |
CORMA A, CONCEPCION P, SERNA P. A different reaction pathway for the reduction of aromatic nitro compounds on gold catalysts[J]. Angewandte Chemie-International Edition, 2007, 46(38): 7266-7269. DOI:10.1002/anie.200700823 |
| [16] |
CORMA A, SERNA P, CONCEPCION P, et al. Transforming nonselective into chemoselective metal catalysts for the hydrogenation of substituted nitroaromatics[J]. Journal of the American Chemical Society, 2008, 130(27): 8748-8753. DOI:10.1021/ja800959g |
| [17] |
TORRES C, CAMPOS C, FIERRO J L G, et al. Nitrobenzene hydrogenation on Au/TiO2 and Au/SiO2 catalyst: Synthesis, characterization and catalytic activity[J]. Catalysis Letters, 2013, 143(8): 763-771. DOI:10.1007/s10562-013-1034-2 |
| [18] |
TRANDAFIR M M, MORAGUES A, AMOROS P, et al. Selective hydrogenation of nitroderivatives over Au/TiO2/UVM-7 composite catalyst[J]. Catalysis Today, 2020, 355: 893-902. DOI:10.1016/j.cattod.2019.02.053 |
| [19] |
TAMIOLAKIS I, FOUNTOULAKI S, VORDOS N, et al. Mesoporous Au-TiO2 nanoparticle assemblies as efficient catalysts for the chemoselective reduction of nitro compounds[J]. Journal of Materials Chemistry A, 2013, 1(45): 14311-14319. DOI:10.1039/c3ta13365f |
| [20] |
YOSHIDA H, IGARASHI N, FUJITA S I, et al. Influence of crystallite size of TiO2 supports on the activity of dispersed Pt catalysts in liquid-phase selective hydrogenation of 3-nitrostyrene, nitrobenzene, and styrene[J]. Catalysis Letters, 2015, 145(2): 606-611. DOI:10.1007/s10562-014-1404-4 |
| [21] |
CARRUS M, FANTAUZZI M, RIBONI F, et al. Increased conversion and selectivity of 4-nitrostyrene hydrogenation to 4-aminostyrene on Pt nanoparticles supported on titanium-tungsten mixed oxides[J]. Applied Catalysis A-General, 2016, 519: 130-138. DOI:10.1016/j.apcata.2016.03.031 |
| [22] |
MACINO M, BARNES A J, QU R Y, et al. Tuning of catalytic sites in Pt/TiO2 catalysts for the chemoselective hydrogenation of 3-nitrostyrene[J]. Nature Catalysis, 2019, 2(10): 873-881. DOI:10.1038/s41929-019-0334-3 |
| [23] |
ZHANG J, PEI L J, WANG J, et al. Differences in the selective reduction mechanism of 4-nitroacetophenone catalysed by rutile- and anatase-supported ruthenium catalysts[J]. Catalysis Science & Technology, 2020, 10(5): 1518-1528. |
| [24] |
ZHANG F W, JIN J, ZHONG X, et al. Pd immobilized on amine-functionalized magnetite nanoparticles: A novel and highly active catalyst for hydrogenation and heck reactions[J]. Green Chemistry, 2011, 13(5): 1238-1243. DOI:10.1039/c0gc00854k |
| [25] |
LONG Y, YUAN B, NIU J R, et al. Distinctive size effects of Pt nanoparticles immobilized on Fe3O4@ppy used as an efficient recyclable catalyst for benzylic alcohol aerobic oxidation and hydrogenation reduction of nitroaromatics[J]. New Journal of Chemistry, 2015, 39(2): 1179-1185. DOI:10.1039/C4NJ01869A |
| [26] |
TAMURA M, YUASA N, NAKAGAWA Y, et al. Selective hydrogenation of nitroarenes to aminoarenes using a MoOx-modified Ru/SiO2 catalyst under mild conditions[J]. Chemical Communications, 2017, 53(23): 3377-3380. DOI:10.1039/C7CC00653E |
| [27] |
WANG L, GUAN E J, ZHANG J, et al. Single-site catalyst promoters accelerate metal-catalyzed nitroarene hydrogenation[J]. Nature Communications, 2018, 9: 1362. DOI:10.1038/s41467-018-03810-y |
| [28] |
ZHANG Q F, SU C, CEN J, et al. The modification of diphenyl sulfide to Pd/C catalyst and its application in selective hydrogenation of p-chloronitrobenzene[J]. Chinese Journal of Chemical Engineering, 2014, 22(10): 1111-1116. DOI:10.1016/j.cjche.2014.08.007 |
| [29] |
BONDARENKO G N, BELETSKAYA I P. Activated carbon as an efficient support for gold nanoparticles that catalyze the hydrogenation of nitro compounds with molecular hydrogen[J]. Mendeleev Communications, 2015, 25(6): 443-445. DOI:10.1016/j.mencom.2015.11.015 |
| [30] |
YUE S N, WANG X G, LI S T, et al. Highly selective hydrogenation of halogenated nitroarenes over Ru/CN nanocomposites by in situ pyrolysis[J]. New Journal of Chemistry, 2020, 44(27): 11861-11869. DOI:10.1039/D0NJ02165B |
| [31] |
LI H B, LIU L, MA X Y. Effective hydrogenation of haloaromatic nitro compounds catalysed by iridium nanoparticles deposited on multiwall carbon nanotubes[J]. Synthesis and Reactivity in Inorganic Metal-Organic and Nano-Metal Chemistry, 2016, 46(10): 1499-1505. DOI:10.1080/15533174.2015.1137013 |
| [32] |
SHENG Y, LIN X R, WANG X G, et al. Highly dispersed Pt nanoparticles on N-doped ordered mesoporous carbon as effective catalysts for selective hydrogenation of nitroarenes[J]. Catalysts, 2020, 10(4): 374. DOI:10.3390/catal10040374 |
| [33] |
ANTONETTI C, OUBENALI M, GALLETTI A M R, et al. Novel microwave synthesis of ruthenium nanoparticles supported on carbon nanotubes active in the selective hydrogenation of p-chloronitrobenzene to p-chloroaniline[J]. Applied Catalysis A-General, 2012, 421: 99-107. |
| [34] |
LU C S, WANG M J, FENG Z L, et al. A phosphorus-carbon framework over activated carbon supported palladium nanoparticles for the chemoselective hydrogenation of parac-hloronitrobenzene[J]. Catalysis Science & Technology, 2017, 7(7): 1581-1589. |
| [35] |
WU Q F, ZHANG B, ZHANG C, et al. Significance of surface oxygen-containing groups and heteroatom P species in switching the selectivity of Pt/C catalyst in hydrogenation of 3-nitrostyrene[J]. Journal of Catalysis, 2018, 364: 297-307. DOI:10.1016/j.jcat.2018.05.025 |
| [36] |
SHU Y J, CHAN H C, XIE L F, et al. Bimetallic platinum-tin nanoparticles on hydrogenated molybdenum oxide for the selective hydrogenation of functionalized nitroarenes[J]. ChemCatChem, 2017, 9(22): 4199-4205. DOI:10.1002/cctc.201700880 |
| [37] |
CAI S F, DUAN H H, RONG H P, et al. Highly active and selective catalysis of bimetallic Rh3Ni1 nanoparticles in the hydrogenation of nitroarenes[J]. ACS Catalysis, 2013, 3(4): 608-612. DOI:10.1021/cs300689w |
| [38] |
LANG L M, PAN Z R, YAN J. Ni-Au alloy nanoparticles as a high performance heterogeneous catalyst for hydrogenation of aromatic nitro compounds[J]. Journal of Alloys and Compounds, 2019, 792: 286-290. DOI:10.1016/j.jallcom.2019.03.323 |
| [39] |
TEGEDER P, FREITAG M, CHEPIGA K M, et al. N-heterocyclic carbene-modified Au-Pd alloy nanoparticles and their application as biomimetic and heterogeneous catalysts[J]. Chemistry-A European Journal, 2018, 24(70): 18682-18688. DOI:10.1002/chem.201803274 |
| [40] |
BUSTAMANTE T M, CAMPOS C H, FRAGA M A, et al. Promotional effect of palladium in Co-SiO2 core@shell nanocatalysts for selective liquid phase hydrogenation of chloronitroarenes[J]. Journal of Catalysis, 2020, 385: 224-237. DOI:10.1016/j.jcat.2020.03.006 |
| [41] |
FURUKAWA S, TAKAHASHI K, KOMATSU T. Well-structured bimetallic surface capable of molecular recognition for chemoselective nitroarene hydrogenation[J]. Chemical Science, 2016, 7(7): 4476-4484. DOI:10.1039/C6SC00817H |
| [42] |
LI X, WANG Y, LI L Q, et al. Deficient copper decorated platinum nanoparticles for selective hydrogenation of chloronitrobenzene[J]. Journal of Materials Chemistry A, 2017, 5(22): 11294-11300. DOI:10.1039/C7TA01587A |
| [43] |
WU Q F, ZHANG C, LIN W W, et al. Selective hydrogenation of 3-nitrostyrene over a Co-promoted Pt catalyst supported on P-containing activated charcoal[J]. Catalysts, 2019, 9(5): 428. DOI:10.3390/catal9050428 |
| [44] |
IIHAMA S, FURUKAWA S, KOMATSU T. Efficient catalytic system for chemoselective hydrogenation of halonitrobenzene to haloaniline using PtZn intermetallic compound[J]. ACS Catalysis, 2016, 6(2): 742-746. DOI:10.1021/acscatal.5b02464 |
| [45] |
MAO J J, CHEN W X, SUN W M, et al. Rational control of the selectivity of a ruthenium catalyst for hydrogenation of 4-nitrostyrene by strain regulation[J]. Angewandte Chemie-International Edition, 2017, 56(39): 11971-11975. DOI:10.1002/anie.201706645 |
| [46] |
PEI Y C, QI Z Y, GOH T W, et al. Intermetallic structures with atomic precision for selective hydrogenation of nitroarenes[J]. Journal of Catalysis, 2017, 356: 307-314. DOI:10.1016/j.jcat.2017.10.011 |
| [47] |
HAN A J, ZHANG J, SUN W M, et al. Isolating contiguous Pt atoms and forming Pt-Zn intermetallic nanoparticles to regulate selectivity in 4-nitrophenylacetylene hydrogenation[J]. Nature Communications, 2019, 10: 3787. DOI:10.1038/s41467-019-11794-6 |
| [48] |
QIAO B T, WANG A Q, YANG X F, et al. Single-atom catalysis of Co oxidation using Pt-1/FeOx[J]. Nature Chemistry, 2011, 3(8): 634-641. DOI:10.1038/nchem.1095 |
| [49] |
WEI H S, REN Y J, WANG A Q, et al. Remarkable effect of alkalis on the chemoselective hydrogenation of functionalized nitroarenes over high-loading P/FeOx catalysts[J]. Chemical Science, 2017, 8(7): 5126-5131. DOI:10.1039/C7SC00568G |
| [50] |
SHI X X, WANG X G, SHANG X F, et al. High performance and active sites of a ceria-supported palladium catalyst for solvent-free chemoselective hydrogenation of nitroarenes[J]. ChemCatChem, 2017, 9(19): 3743-3751. DOI:10.1002/cctc.201700631 |
| [51] |
PENG Y H, GENG Z G, ZHAO S T, et al. Pt single atoms embedded in the surface of Ni nanocrystals as highly active catalysts for selective hydrogenation of nitro compounds[J]. Nano Letters, 2018, 18(6): 3785-3791. DOI:10.1021/acs.nanolett.8b01059 |
| [52] |
LIN L L, YAO S Y, GAO R, et al. A highly CO-tolerant atomically dispersed Pt catalyst for chemoselective hydrogenation[J]. Nature Nanotechnology, 2019, 14(4): 354-361. DOI:10.1038/s41565-019-0366-5 |
| [53] |
CHEN G X, XU C F, HUANG X Q, et al. Interfacial electronic effects control the reaction selectivity of platinum catalysts[J]. Nature Materials, 2016, 15(5): 564-569. DOI:10.1038/nmat4555 |
| [54] |
WANG Y, QIN R X, WANG Y K, et al. Chemoselective hydrogenation of nitroaromatics at the nanoscale iron(III)-OH-platinum interface[J]. Angewandte Chemie-International Edition, 2020, 59(31): 12736-12740. DOI:10.1002/anie.202003651 |
| [55] |
GU J, ZHANG Z Y, HU P, et al. Platinum nanoparticles encapsulated in MFI zeolite crystals by a two-step dry gel conversion method as a highly selective hydrogenation catalyst[J]. ACS Catalysis, 2015, 5(11): 6893-6901. DOI:10.1021/acscatal.5b01823 |
| [56] |
CHEN Q, WANG M Y, ZHANG C X, et al. Selectivity control on hydrogenation of substituted nitroarenes through end-on adsorption of reactants in zeolite-encapsulated platinum nanoparticles[J]. Chemistry-An Asian Journal, 2018, 13(16): 2077-2084. DOI:10.1002/asia.201800596 |
| [57] |
ZHANG J, WANG L, SHAO Y, et al. A Pd@zeolite catalyst for nitroarene hydrogenation with high product selectivity by sterically controlled adsorption in the zeolite micropores[J]. Angewandte Chemie-International Edition, 2017, 56(33): 9747-9751. DOI:10.1002/anie.201703938 |
| [58] |
YAN X R, CHEN L L, SONG H X, et al. Metal–organic framework (MOF)-derived catalysts for chemoselective hydrogenation of nitroarenes[J]. New Journal of Chemistry, 2021, 45(39): 18268-18276. DOI:10.1039/D1NJ03227E |
| [59] |
WESTERHAUS F A, JAGADEESH R V, WIENHOEFER G, et al. Heterogenized cobalt oxide catalysts for nitroarene reduction by pyrolysis of molecularly defined complexes[J]. Nature Chemistry, 2013, 5(6): 537-543. DOI:10.1038/nchem.1645 |
| [60] |
PISIEWICZ S, FORMENTI D, SURKUS A E, et al. Synthesis of nickel nanoparticles with N-doped graphene shells for catalytic reduction reactions[J]. ChemCatChem, 2016, 8(1): 129-134. DOI:10.1002/cctc.201500848 |
| [61] |
JAGADEESH R V, SURKUS A E, JUNGE H, et al. Nanoscale Fe2O3-based catalysts for selective hydrogenation of nitroarenes to anilines[J]. Science, 2013, 342(6162): 1073-1076. DOI:10.1126/science.1242005 |
| [62] |
FORMENTI D, FERRETTI F, TOPF C, et al. Co-based heterogeneous catalysts from well-defined alpha-diimine complexes: Discussing the role of nitrogen[J]. Journal of Catalysis, 2017, 351: 79-89. DOI:10.1016/j.jcat.2017.04.014 |
| [63] |
SAHOO B, FORMENTI D, TOPF C, et al. Biomass-derived catalysts for selective hydrogenation of nitroarenes[J]. ChemSusChem, 2017, 10(15): 3035-3039. DOI:10.1002/cssc.201700796 |
| [64] |
WEI Z Z, WANG J, MAO S J, et al. In situ-generated Co-0-Co3O4/N-doped carbon nanotubes hybrids as efficient and chemoselective catalysts for hydrogenation of nitroarenes[J]. ACS Catalysis, 2015, 5(8): 4783-4789. DOI:10.1021/acscatal.5b00737 |
| [65] |
CHEN P R, YANG F K, KOSTKA A, et al. Interaction of cobalt nanoparticles with oxygen- and nitrogen-functionalized carbon nanotubes and impact on nitrobenzene hydrogenation catalysis[J]. ACS Catalysis, 2014, 4(5): 1478-1486. DOI:10.1021/cs500173t |
| [66] |
CUI X L, LIANG K, TIAN M, et al. Cobalt nanoparticles supported on N-doped mesoporous carbon as a highly efficient catalyst for the synthesis of aromatic amines[J]. Journal of Colloid and Interface Science, 2017, 501: 231-240. DOI:10.1016/j.jcis.2017.04.053 |
| [67] |
ZHOU P, JIANG L, WANG F, et al. High performance of a cobalt-nitrogen complex for the reduction and reductive coupling of nitro compounds into amines and their derivatives[J]. Science Advances, 2017, 3(2): e1601945. DOI:10.1126/sciadv.1601945 |
| [68] |
BARAMOV T, LOOS P, HASSFELD J, et al. Encapsulated cobalt oxide on carbon nanotube support as catalyst for selective continuous hydrogenation of the showcase substrate 1-iodo-4-nitrobenzene[J]. Advanced Synthesis & Catalysis, 2016, 358(18): 2903-2911. |
| [69] |
MA X, ZHOU Y X, LIU H, et al. A mof-derived Co-CoO@N-doped porous carbon for efficient tandem catalysis: Dehydrogenation of ammonia borane and hydrogenation of nitro compounds[J]. Chemical Communications, 2016, 52(49): 7719-7722. DOI:10.1039/C6CC03149H |
| [70] |
YANG S L, PENG L, OVEISI E, et al. MOF-derived cobalt phosphide/carbon nanocubes for selective hydrogenation of nitroarenes to anilines[J]. Chemistry - A European Journal, 2018, 24(17): 4234-4238. DOI:10.1002/chem.201705400 |
| [71] |
YANG S L, PENG L, SUN D T, et al. Metal–organic-framework-derived Co3S4 hollow nanoboxes for the selective reduction of nitroarenes[J]. ChemSusChem, 2018, 11(18): 3131-3138. DOI:10.1002/cssc.201801641 |
| [72] |
WANG X, LI Y W. Chemoselective hydrogenation of functionalized nitroarenes using mof-derived Co-based catalysts[J]. Journal of Molecular Catalysis A-Chemical, 2016, 420: 56-65. DOI:10.1016/j.molcata.2016.04.008 |
| [73] |
HU A, LU X H, CAI D M, et al. Selective hydrogenation of nitroarenes over mof-derived Co@CN catalysts at mild conditions[J]. Molecular Catalysis, 2019, 472: 27-36. DOI:10.1016/j.mcat.2019.04.008 |
| [74] |
WANG H J, WANG Y, LI Y F, et al. Highly efficient hydrogenation of nitroarenes by N-doped carbon-supported cobalt single-atom catalyst in ethanol/water mixed solvent[J]. ACS Applied Materials & Interfaces, 2020, 12(30): 34021-34031. |
| [75] |
LAN X C, ALI B, WANG Y, et al. Hollow and yolk-shell Co-N-C@SiO2 nanoreactors: Controllable synthesis with high selectivity and activity for nitroarene hydrogenation[J]. ACS Applied Materials & Interfaces, 2020, 12(3): 3624-3630. |
| [76] |
SUN X, OLIVOS-SUAREZ A I, OSADCHII D, et al. Single cobalt sites in mesoporous N-doped carbon matrix for selective catalytic hydrogenation of nitroarenes[J]. Journal of Catalysis, 2018, 357: 20-28. DOI:10.1016/j.jcat.2017.10.030 |
| [77] |
LI M H, CHEN S Y, JIANG Q K, et al. Origin of the activity of Co-N-C catalysts for chemoselective hydrogenation of nitroarenes[J]. ACS Catalysis, 2021, 11(5): 3026-3039. DOI:10.1021/acscatal.0c05479 |
| [78] |
SORRIBES I, LIU L C, CORMA A. Nanolayered Co-Mo-S catalysts for the chemoselective hydrogenation of nitroarenes[J]. ACS Catalysis, 2017, 7(4): 2698-2708. DOI:10.1021/acscatal.7b00170 |
| [79] |
ZHANG G J, TANG F Y, WANG X Y, et al. Co, N-codoped porous carbon-supported coyzns with superior activity for nitroarene hydrogenation[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(15): 6118-6126. |
| [80] |
LU C S, LV J H, MA L, et al. Highly selective hydrogenation of halonitroaromatics to aromatic haloamines by ligand modified Ni-based catalysts[J]. Chinese Chemical Letters, 2012, 23(5): 545-548. DOI:10.1016/j.cclet.2012.03.017 |
| [81] |
LIU X, MA X X, LIU S J, et al. Metal fluoride promoted catalytic hydrogenation of aromatic nitro compounds over Raney®Ni[J]. RSC Advances, 2015, 5(46): 36423-36427. DOI:10.1039/C5RA02725J |
| [82] |
LI H, ZHANG J, LI H X. Ultrasound-assisted preparation of a novel Ni-B amorphous catalyst in uniform nanoparticles for p-chloronitrobenzene hydrogenation[J]. Catalysis Communications, 2007, 8(12): 2212-2216. DOI:10.1016/j.catcom.2007.05.006 |
| [83] |
LI H, ZHAO Q F, LI H X. Selective hydrogenation of p-chloronitrobenzene over Ni-P-B amorphous catalyst and synergistic promoting effects of B and P[J]. Journal of Molecular Catalysis A-Chemical, 2008, 285(1-2): 29-35. DOI:10.1016/j.molcata.2008.01.025 |
| [84] |
HAHN G, EWERT J K, DENNER C, et al. A reusable mesoporous nickel nanocomposite catalyst for the selective hydrogenation of nitroarenes in the presence of sensitive functional groups[J]. ChemCatChem, 2016, 8(15): 2461-2465. DOI:10.1002/cctc.201600391 |
| [85] |
QU Y M, YANG H, WANG S L, et al. Hydrogenation of nitrobenzene to aniline catalyzed by C60-stabilized Ni[J]. Catalysis Communications, 2017, 97: 83-87. DOI:10.1016/j.catcom.2017.04.029 |
| [86] |
LU X H, WEI X L, ZHOU D, et al. Synthesis, structure and catalytic activity of the supported Ni catalysts for highly efficient one-step hydrogenation of 1, 5-dinitronaphthalene to 1, 5-diaminodecahydronaphthalene[J]. Journal of Molecular Catalysis A-Chemical, 2015, 396: 196-206. DOI:10.1016/j.molcata.2014.08.030 |
| [87] |
LI Y Z, YU J Y, LI W, et al. The promotional effect of surface defects on the catalytic performance of supported nickel-based catalysts[J]. Physical Chemistry Chemical Physics, 2016, 18(9): 6548-6558. DOI:10.1039/C5CP07371E |
| [88] |
REN Y J, WEI H S, YIN G Z, et al. Oxygen surface groups of activated carbon steer the chemoselective hydrogenation of substituted nitroarenes over nickel nanoparticles[J]. Chemical Communications, 2017, 53(12): 1969-1972. DOI:10.1039/C6CC08505A |
| [89] |
HUANG L, LV Y, LIU S, et al. Non-noble metal Ni nanoparticles supported on highly dispersed TiO2-modified activated carbon as an efficient and recyclable catalyst for the hydrogenation of halogenated aromatic nitro compounds under mild conditions[J]. Industrial & Engineering Chemistry Research, 2020, 59(4): 1422-1435. |
| [90] |
HUANG H G, WANG X G, SHENG Y, et al. Nitrogen-doped graphene-activated metallic nanoparticle-incorporated ordered mesoporous carbon nanocomposites for the hydrogenation of nitroarenes[J]. RSC Advances, 2018, 8(16): 8898-8909. DOI:10.1039/C8RA00761F |
| [91] |
DUTTA D, DUTTA D K. Selective and efficient hydrogenation of halonitrobenzene catalyzed by clay supported Ni-O-nanoparticles[J]. Applied Catalysis A-General, 2014, 487: 158-164. DOI:10.1016/j.apcata.2014.09.004 |
| [92] |
XIONG W, WANG L P, CAI G X, et al. Nitrogen-functionalized active carbon-supported non-noble nickel nanoparticles with high dispersity and enhanced catalytic performance in nitro naphthalene hydrogenation[J]. ChemistrySelect, 2017, 2(34): 11244-11249. DOI:10.1002/slct.201702093 |
| [93] |
ADVANI J H, RAVI K, NAIKWADI D R, et al. Bio-waste chitosan-derived N-doped cnt-supported Ni nanoparticles for selective hydrogenation of nitroarenes[J]. Dalton Transactions, 2020, 49(30): 10431-10440. DOI:10.1039/D0DT01708F |
| [94] |
RYABCHUK P, AGOSTINI G, POHL M M, et al. Intermetallic nickel silicide nanocatalyst-A non-noble metal-based general hydrogenation catalyst[J]. Science Advances, 2018, 4(6): eaat0761. DOI:10.1126/sciadv.aat0761 |
| [95] |
CHEN J, YAO Y, ZHAO J, et al. A highly active non-precious metal catalyst based on Fe-N-C@CNTs for nitroarene reduction[J]. RSC Advances, 2016, 6(98): 96203-96209. DOI:10.1039/C6RA20666B |
| [96] |
XU S D, YU D Q, LIAO S F, et al. Nitrogen-doped carbon supported iron oxide as efficient catalysts for chemoselective hydrogenation of nitroarenes[J]. RSC Advances, 2016, 6(98): 96431-96435. DOI:10.1039/C6RA18935K |
| [97] |
WANG Y Y, SHI J J, ZHANG Z H, et al. Carbon film encapsulated Fe2O3: An efficient catalyst for hydrogenation of nitroarenes[J]. Chinese Journal of Catalysis, 2017, 38(11): 1909-1917. DOI:10.1016/S1872-2067(17)62917-6 |
| [98] |
NIU H L, LU J H, SONG J J, et al. Iron oxide as a catalyst for nitroarene hydrogenation: Important role of oxygen vacancies[J]. Industrial & Engineering Chemistry Research, 2016, 55(31): 8527-8533. |
| [99] |
JAGADEESH R V, STEMMLER T, SURKUS A E, et al. Hydrogenation using iron oxide-based nanocatalysts for the synthesis of amines[J]. Nature Protocols, 2015, 10(4): 548-557. DOI:10.1038/nprot.2015.025 |
| [100] |
SHI J J, WANG Y Y, DU W C, et al. Synthesis of graphene encapsulated Fe3C in carbon nanotubes from biomass and its catalysis application[J]. Carbon, 2016, 99: 330-337. DOI:10.1016/j.carbon.2015.12.049 |
| [101] |
MA B, TONG X L, GUO C X, et al. Pyrite nanoparticles: An earth-abundant mineral catalyst for activation of molecular hydrogen and hydrogenation of nitroaromatics[J]. RSC Advances, 2016, 6(60): 55220-55224. DOI:10.1039/C6RA10785K |
| [102] |
MORSE J R, CALLEJAS J F, DARLING A J, et al. Bulk iron pyrite as a catalyst for the selective hydrogenation of nitroarenes[J]. Chemical Communications, 2017, 53(35): 4807-4810. DOI:10.1039/C7CC00120G |
| [103] |
ZHU Y A, YANG S L, CAO C Y, et al. Controllable synthesis of carbon encapsulated iron phosphide nanoparticles for the chemoselective hydrogenation of aromatic nitroarenes to anilines[J]. Inorganic Chemistry Frontiers, 2018, 5(5): 1094-1099. DOI:10.1039/C7QI00803A |
| [104] |
DUAN Y N, DONG X S, SONG T, et al. Hydrogenation of functionalized nitroarenes catalyzed by single-phase pyrite FeS2 nanoparticles on N, S-codoped porous carbon[J]. ChemSusChem, 2019, 12(20): 4636-4644. DOI:10.1002/cssc.201901867 |
| [105] |
YE T N, LU Y F, LI J, et al. Copper-based intermetallic electride catalyst for chemoselective hydrogenation reactions[J]. Journal of the American Chemical Society, 2017, 139(47): 17089-17097. DOI:10.1021/jacs.7b08252 |
| [106] |
KOUR G, GUPTA M, VISHWANATHAN B, et al. (Cu/NCNTs): A new high temperature technique to prepare a recyclable nanocatalyst for four component pyridine derivative synthesis and nitroarenes reduction[J]. New Journal of Chemistry, 2016, 40(10): 8535-8542. DOI:10.1039/C6NJ01464J |
| [107] |
NI T, ZHANG S, CAO F X, et al. NiCo bimetallic nanoparticles encapsulated in graphite-like carbon layers as efficient and robust hydrogenation catalysts[J]. Inorganic Chemistry Frontiers, 2017, 4(12): 2005-2011. DOI:10.1039/C7QI00492C |
| [108] |
SERNA P, CORMA A. Transforming nano metal nonselective particulates into chemoselective catalysts for hydrogenation of substituted nitrobenzenes[J]. ACS Catalysis, 2015, 5(12): 7114-7121. DOI:10.1021/acscatal.5b01846 |
| [109] |
LIU L C, GAO F, CONCEPCION P, et al. A new strategy to transform MonO and bimetallic non-noble metal nanoparticles into highly active and chemoselective hydrogenation catalysts[J]. Journal of Catalysis, 2017, 350: 218-225. DOI:10.1016/j.jcat.2017.03.014 |
| [110] |
HAN B H, SHIN D H, CHO S Y. Graphite catalyzed reduction of aromatic and aliphatic nitro-compounds with hydrazine hydrate[J]. Tetrahedron Letters, 1985, 26(50): 6233-6234. DOI:10.1016/S0040-4039(00)95060-3 |
| [111] |
SCHIMMEL H G, KEARLEY G J, NIJKAMP M G, et al. Hydrogen adsorption in carbon nanostructures: Comparison of nanotubes, fibers, and coals[J]. Chemistry-A European Journal, 2003, 9(19): 4764-4770. DOI:10.1002/chem.200304845 |
| [112] |
CHEN X H, SHEN Q J, LI Z J, et al. Metal-free h-2 activation for highly selective hydrogenation of nitroaromatics using phosphorus-doped carbon nanotubes[J]. ACS Applied Materials & Interfaces, 2020, 12(1): 654-666. |
| [113] |
SHANG S S, GAO S. Heteroatom-enhanced metal-free catalytic performance of carbocatalysts for organic transformations[J]. ChemCatChem, 2019, 11(16): 3728-3742. |
| [114] |
PRIMO A, NEATU F, FLOREA M, et al. Graphenes in the absence of metals as carbocatalysts for selective acetylene hydrogenation and alkene hydrogenation[J]. Nature Communications, 2014, 5: 5291. DOI:10.1038/ncomms6291 |
| [115] |
LUO Z C, NIE R F, NGUYEN V T, et al. Transition metal-like carbocatalyst[J]. Nature Communications, 2020, 11(1): 4091. DOI:10.1038/s41467-020-17909-8 |
| [116] |
GAO R J, PAN L, LU J H, et al. Phosphorus-doped and lattice-defective carbon as metal-like catalyst for the selective hydrogenation of nitroarenes[J]. ChemCatChem, 2017, 9(22): 4287-4294. DOI:10.1002/cctc.201700904 |
| [117] |
CHEN P R, CHEW L M, XIA W. The influence of the residual growth catalyst in functionalized carbon nanotubes on supported Pt nanoparticles applied in selective olefin hydrogenation[J]. Journal of Catalysis, 2013, 307: 84-93. DOI:10.1016/j.jcat.2013.06.030 |
| [118] |
WU S C, WEN G D, WANG J, et al. Nitrobenzene reduction catalyzed by carbon: Does the reaction really belong to carbocatalysis?[J]. Catalysis Science & Technology, 2014, 4(12): 4183-4187. |
| [119] |
LI B J, XU Z. A nonmetal catalyst for molecular hydrogen activation with comparable catalytic hydrogenation capability to noble metal catalyst[J]. Journal of the American Chemical Society, 2009, 131(45): 16380-16382. DOI:10.1021/ja9061097 |
| [120] |
PACOSOVA L, KARTUSCH C, KUKULA P, et al. Is fullerene a nonmetal catalyst in the hydrogenation of nitrobenzene?[J]. ChemCatChem, 2011, 3(1): 154-156. DOI:10.1002/cctc.201000229 |