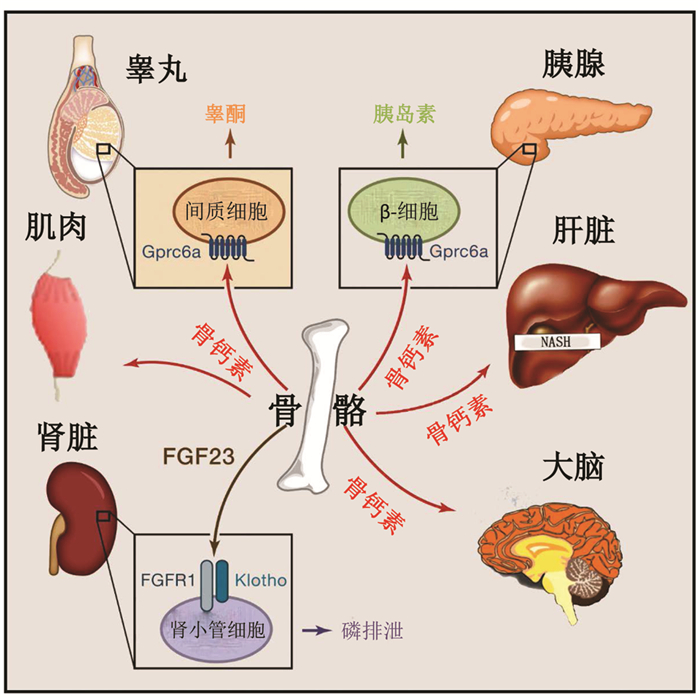
内分泌是指机体产生的生物活性物质可不通过导管而直接分泌入血的现象,与分泌物经导管排出体外或引至体内其他部位的外分泌相对应。传统意义上,经典内分泌器官指的是甲状腺、性腺和肾上腺等。然而,一些非经典的内分泌器官同样具有内分泌功能[1]。骨骼便是其中一种被忽视的重要内分泌器官,它可与系统器官诸如肌肉、大脑、肾脏、生殖腺、胰腺、肝脏等通过特殊的生物活性物质进行信息传递,密切对话。
骨骼与肌肉的对话骨质疏松症和肌少症常相伴出现。Frost等[2]提出机械负荷理论解释此现象,认为骨骼主要受其相邻肌肉体积和肌肉活动的调节,具有根据肌肉施加机械负荷变化而适应性改变骨骼形状和强度的特性,即骨骼可根据肌肉施加机械负荷的大小来适应性调节其骨结构和骨量,当机械力超过生理应力时促骨形成,低于时则促骨吸收。正如研究发现,体育锻炼时,肌肉收缩对骨骼施加机械应力,可增加骨骼强度[3];而肌麻痹时,骨骼由于长期缺乏应力刺激,骨干形状变为圆形,骨结构改变,骨质疏松风险增加[4]。
但是,机械负荷理论却不能很好地解释以下现象。如Devan等[5]发现开放性胫骨骨折创面覆盖肌瓣可以加速骨折愈合,Landry等[6]也发现骨缺损周围存在肌肉损伤时,骨再生延迟。事实上,骨骼和肌肉之间除了存在前述机械力学上的耦联,还存在生物化学上的关联,骨骼和肌肉均可作为内分泌器官,分别通过释放骨源性因子和肌源性因子,调节彼此的功能[7]。
肌肉可分泌多种因子作用于骨骼,如鸢尾素、肌生成抑制蛋白等。鸢尾素可激活成骨细胞活性,在增加骨桥蛋白的同时,减少骨硬化蛋白,促骨形成[8]。肌生成抑制蛋白具有促进破骨,抑制骨修复的作用[9]。骨骼也可分泌多种因子影响肌肉,如骨钙素、前列腺素E2(prostaglandin E2,PGE2)、Wnt3a蛋白等。Mera等[10-11]认为骨钙素可直接作用于肌肉的G蛋白耦联受体C家族6型A(G protein-coupled receptor class C group 6 member A,GPRC6A)受体,通过促进肌纤维吸收和利用营养来调节肌肉功能,锻炼前注射骨钙素,幼龄鼠的运动能力增强,老龄鼠肌肉体积增加,有氧耐力得以恢复。PGE2和Wnt3a蛋白具有促进肌肉生成并维持肌肉功能的作用[12-13]。
骨骼和肌肉存在紧密对话,参与对话的鸢尾素、肌生成抑制蛋白、骨钙素等将来可作为同时治疗骨质疏松症和肌少症的靶点。
骨骼与脑的对话脑疾病可影响骨骼。如阿尔茨海默病(Alzheimer's disease,AD)患者较同龄的正常人骨质疏松发病率更高;重度抑郁症、精神分裂症等也常与骨量降低相关。此外,骨骼疾病也会影响大脑。如颅骨锁骨发育不全综合征、复杂性局部疼痛综合征等被证实与大脑认知损害相关联[14]。
脑疾病和骨骼疾病的紧密联系提示脑和骨骼之间存在密切的对话。事实上,大脑可通过极其复杂的神经通路调节骨骼代谢。脂肪细胞分泌的瘦素通过外周和中枢对骨骼的作用截然相反,一方面外周瘦素可直接结合并激活成骨细胞上的瘦素受体,发挥促成骨作用[15];而另一方面中枢瘦素可作用于脑干和下丘脑不同部位的瘦素受体,激活交感神经系统活性,释放儿茶酚胺,进一步作用于成骨细胞上的肾上腺素能受体,抑制成骨活性,降低骨量[16]。Bajayo等[17]研究报道除了交感神经可对骨量调节外,副交感神经也可通过节前神经元分泌乙酰胆碱的方式,拮抗交感神经,增加骨量,并发现白介素1-副交感神经系统-骨骼轴在骨量的正向调节中扮演重要的角色。Fukuda等[18]发现感觉神经系统通过信号素3A(semaphorin3a,Sema3A)也参与了骨量的正向调节。Deng等[19]随后又进一步报道信号素4D(semaphorin4d,Sema4D)以及信号素6D(semaphorin6d,Sema6D)具有类似Sema3A的作用。此外,一些神经递质如神经肽、神经调节肽U、脑源性神经营养因子(brain-derived neurotro-phic factor,BDNF)等对骨骼也具有重要的调节作用[14]。
骨骼也可分泌骨钙素和脂质运载蛋白2(lipocalin 2,LCN2)等影响大脑的功能[20-21]。Oury等[20]发现骨钙素缺乏的鼠常表现焦虑样行为增加、学习和记忆能力下降。并且,骨钙素缺乏鼠与正常鼠相比,大脑更小,海马的齿状回区域面积明显缩小达30%,连接两侧海马半球的胼胝体消失。Wise等[22]和Ende[23]认为骨钙素发挥作用可能与神经递质变化有关。因为骨钙素缺乏鼠较正常鼠相比,5-羟色胺、多巴胺和去甲肾上腺素下降20%~50%,而抑制性神经递质γ-氨基丁酸增加15%~30%。Khrimian等[24]后来进一步研究发现骨钙素可穿过血-脑脊液屏障,作用于大脑特定区域(如海马、中脑腹侧被盖区、黑质区和脑干等)上的G蛋白偶联受体158(G protein-coupled receptor158,GPR158),增加BDNF的表达来调节大脑认知。Oury等[20]还发现胚胎期鼠大脑的发育大部分依赖母亲来源的骨钙素,骨钙素可通过胎盘屏障进入胎儿体内,骨钙素缺乏会不同程度影响胎儿的神经发育。
Mosialou等[21]发现主要由骨骼中成骨细胞分泌的LCN2,也能通过血-脑脊液屏障,结合并激活下丘脑中的黑素皮质激素4受体(melanocortin 4 receptor,MC4R),抑制食欲。
骨骼与肾脏的对话肾脏疾病可影响骨骼。慢性肾脏病-矿物质和骨异常(chronic kidney disease-mineral and bone disorder,CKD-MBD)被认为是由于慢性肾脏病导致的矿物质及骨代谢异常综合征,表现为钙、磷、甲状旁腺素、或维生素D代谢异常,骨转化、矿化、骨量、骨线性生长或骨强度异常[25]。
骨骼也可通过分泌成纤维生长因子23(fibroblast growth factor-23,FGF23)作用于肾脏。FGF23在受体α-Klotho蛋白的参与下,可与肾近端小管上的成纤维细胞生长因子受体1(fibroblast growth factor receptor 1,FGFR1)结合,通过激活细胞外信号调节激酶1/2(extracellular signal-regulated kinase 1/2,EPK1/2)和糖皮质激素诱导的激酶1(glucocorticoid induced kinase 1,SGK1),下调钠依赖性磷酸盐共转运体2a(sodium-phosphate cotransporter 2a,NPT2a)和钠依赖性磷酸盐共转运体2c(sodium-phosphate cotransporter 2c,NPT2c)两种转运蛋白(磷的重吸收主要依靠NPT2a和NPT2c两种转运蛋白),降低血磷水平。此外,FGF23还可通过降低1-α-羟化酶和升高24-羟化酶的表达,下调1,25-二羟维生素D3的合成和活性,而由于1,25-二羟维生素D3可增加肠道对血磷的吸收,FGF23可进一步降低血磷水平。FGFR-α-Klotho复合体在磷和维生素D代谢中发挥重要作用,是骨骼与肾脏对话的重要媒介[26]。
如果对骨骼进行干预,调节FGF23水平或许能为治疗FGF23失衡引起的磷代谢相关疾病提供新的治疗方式。
骨骼与生殖腺的对话睾丸分泌的睾酮和卵巢分泌的雌激素,不仅对生殖功能有重要调节作用,也在骨的生长、成熟和维持骨骼完整性方面发挥重要作用。绝经后妇女可出现骨质疏松,性腺功能障碍可引起骨量丢失等现象均提示生殖腺对骨骼的重要调节作用[27]。
除了通过下丘脑-垂体-性腺轴调节睾丸功能外,骨骼作为内分泌器官,也可通过释放骨钙素调节性腺的生殖功能。Oury等[28]最早发现成骨细胞来源的骨钙素可刺激睾丸间质细胞分泌睾酮。他们发现骨钙素缺乏的雄鼠精巢重量下降,睾丸间质细胞成熟出现停滞,精子数量下降50%。而通过对骨钙素缺乏的鼠补充外源性骨钙素可增加睾酮的生成。实际上,骨钙素可与睾丸间质细胞上的Gprc6A受体结合,通过激活cAMP反应原件结合蛋白(cAMP-response element binding protein,CREB)通路相关酶,增加睾酮的产生。骨钙素对生殖的影响仅限于雄性,并不能升高雌性睾酮或雌激素的水平。
然而有研究报道并没有发现脱羧化骨钙素与精液浓度、精子形态和活性相关,也没有发现脱羧化骨钙素与血清黄体生成素或睾酮水平相关[29]。Barbonetti等[30]认为是下丘脑-垂体-性腺轴的存在掩盖了骨钙素的影响,他们通过制备慢性脊髓损伤模型排除中枢对睾酮的影响,发现骨钙素确实可刺激睾酮的生成。
骨骼与胰腺的对话糖尿病患者晚期糖基化终末产物和同型半胱氨酸增加,可直接破坏成骨细胞和骨细胞的功能,减少骨量,增加骨折风险。因此,骨质疏松症被认为是糖尿病的并发症之一[31]。
Karsenty[32]在2006年首次提出骨骼通过分泌骨钙素,可发挥能量代谢调节作用。Ferron等[33]发现在pH值为4.5左右的酸性环境下,细胞外基质中与羟磷灰石结合的羧化骨钙素可发生脱羧化而活化,脱羧化骨钙素可作用于胰腺和脂肪细胞,对糖代谢进行调节。骨钙素缺乏常会出现血糖升高、胰岛素下降、胰岛素敏感性降低、胰岛缩小、β细胞数量减少,以及内脏脂肪和三酰甘油增加等[34]。
事实上,骨钙素和胰岛素的协同作用,在能量和糖代谢调节中扮演重要角色。Ferron等[33]发现骨骼可作为胰岛素的靶器官,胰岛素可直接作用于成骨细胞上的胰岛素受体,下调骨保护素(osteoprotegerin,OPG)表达,增加破骨细胞活性,促进骨吸收,使细胞外基质酸化,骨钙素在酸性环境下脱羧化激活。
Oldknow等[35]发现交感神经系统激活后,可通过增加成骨细胞活化转录因子4(activiting trans-cription factor 4,ATF4)表达,激活肠球菌表面蛋白(enterococcal surface protein,ESP)基因表达,后者通过编码受体样蛋白酪氨酸磷酸酶(osteotesticular protein tyrosine phosphatase,OST-PTP),参与骨钙素的羧化过程,降低骨钙素活性。
基于肥胖和2型糖尿病患者骨钙素水平都很低,Kindblom等[36]研究提出可以将骨钙素的动态变化作为糖代谢和能量代谢变化的新的判断指标。值得注意的是,使用阿仑膦酸钠和雷洛昔芬治疗骨质疏松症12周后,骨钙素水平下降。抑制骨吸收的药物是否会降低机体糖耐受能力,目前尚不明确[33]。
骨骼与肝脏的对话非酒精性脂肪肝炎(non-alcoholic steatohe-patitis,NASH)是全世界最常见的慢性肝脏疾病之一,现被认为是可影响肝外多器官功能的多系统疾病。NASH会引起骨密度降低。此外,严重肝脏疾病也可能会引起骨矿化能力下降,导致骨软化症[37]。
研究认为,炎性肝脏可能会释放骨桥蛋白(osteopontin,OPN)、肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)、胎球蛋白等细胞因子影响骨代谢。骨桥蛋白不仅可直接作用肝脏,加重NASH的炎性反应和纤维化,其过表达可增加绝经后骨质疏松症的发生率。当骨桥蛋白>14.7 mg/L时可认为是发生绝经后骨质疏松症的一项危险因素[38]。
骨骼也可通过分泌骨钙素与肝脏对话。Yilmaz等发现骨钙素水平与丙氨酸氨基转移酶(alanine transaminase,ALT)和天门冬氨酸氨基转移酶(aspartate transaminase,AST)的浓度成负相关,可作为判断NASH气球样变程度的独立指标[39]。Gupte等[40]研究发现骨钙素可通过减少促炎因子和促纤维化基因的表达,减轻NASH。动物实验也发现皮下注射或间断腹膜内注射骨钙素可以改善肝细胞的脂肪变、气球样变引起的退化和纤维化等[41]。
骨骼和肝脏存在紧密联系,骨桥蛋白、骨钙素等将来可作为同时治疗骨质疏松症和NASH的靶点。
总结骨骼作为内分泌器官,可与系统器官发生密切的对话,协同维持体内的平衡,更好地理解骨骼与系统器官的相互对话及其作用机制,可为治疗系统器官疾病提供新的思路(图 1)。
| [1] | Giacomina B, D'Amelio P, Malgorzata W, et al. Editorial: bone: endocrine target and organ[J]. Front Endocrinol, 2017, 8: 354. DOI:10.3389/fendo.2017.00354 |
| [2] | Frost HM. Bone's mechanosta[J]. Anat Rec A Discov Mol Cell Evol Biol, 2003, 275: 1081–1101. |
| [3] | Sugiyama T. Physical activity and bone health: understanding mechanical strain-related stimuli[J]. Int J Epidemiol, 2018, 47: 669–670. DOI:10.1093/ije/dyy037 |
| [4] | Ausk BJ, Worton LE, Smigiel KS, et al. Muscle Paralysis induces bone marrow inflammation and predisposition to formation of giant osteoclasts[J]. Am J Physiol Cell Physiol, 2017, 313: C533–C540. DOI:10.1152/ajpcell.00363.2016 |
| [5] | Devan M, Salma A, Stranix JT, et al. Comparing radiographic progression of bone healing in gustilo iiib open tibia fractures treated with muscle versus fasciocutaneous flaps[J]. J Orthopaed Trauma, 2018, 32: 381–385. |
| [6] | Landry PS, Marino A, Sadasivan K, et al. Effect of soft-tissue trauma on the early periosteal response of bone to injury[J]. J Trauma, 2000, 48: 479–483. DOI:10.1097/00005373-200003000-00018 |
| [7] | Brotto M, Johnson ML. Endocrine crosstalk between muscle and bone[J]. Curr Osteoporos Rep, 2014, 12: 135–141. DOI:10.1007/s11914-014-0209-0 |
| [8] | Graziana C, Concetta C, Teresa M, et al. The myokine irisin increases cortical bone mass[J]. Proc Natl Acad Sci USA, 2015, 112: 12157–12162. DOI:10.1073/pnas.1516622112 |
| [9] | Wu LF, Zhu DC, Wang BH, et al. Relative abundance of mature myostatin rather than total myostatin is negatively associated with bone mineral density in Chinese[J]. J Cell Mol Med, 2017, 22: 1329–1336. |
| [10] | Mera P, Laue K, Ferron M, et al. Osteocalcin signaling in myofibers is necessary and sufficient for optimum adaptation to exercise[J]. Cell Metab, 2016, 23: 1078–1092. DOI:10.1016/j.cmet.2016.05.004 |
| [11] | Mera P, Laue K, Wei J, et al. Osteocalcin is necessary and sufficient to maintain muscle mass in older mice[J]. Mol Metab, 2016, 5: 1042–1047. DOI:10.1016/j.molmet.2016.07.002 |
| [12] | Mo C, Romero-Suarez S, Bonewald L, et al. Prostaglandin E2: from clinical applications to its potential role in bone- muscle crosstalk and myogenic differentiation[J]. Recent Patents Biotechnol, 2012, 6: 223–229. DOI:10.2174/1872208311206030223 |
| [13] | Huang J, Mo C, Bonewald L, et al. Wnt3a poten-tiates myogenesis in C2C12 myoblasts through the modulation of intracellular calcium and activation of the β-catenin signaling pathway[J]. Faseb J, 2014, 28: 1102–1123. |
| [14] | Rousseaud A, Moriceau S, Ramosbrossier M, et al. Bone-brain crosstalk and potential associated diseases[J]. Hormone Mol Biol Clin Invest, 2016, 28: 69–83. |
| [15] | Turner RT, Kalra SP, Wong CP, et al. Peripheral leptin regulates bone formation[J]. J Bone Mineral Res, 2013, 28: 22–34. DOI:10.1002/jbmr.1734 |
| [16] | Takeda S, Elefteriou F, Levasseur R, et al. Leptin regulates bone formation via the sympathetic nervous system[J]. Cell, 2002, 111: 305–317. DOI:10.1016/S0092-8674(02)01049-8 |
| [17] | Bajayo A, Bar A, Denes A, et al. Skeletal parasympathetic innervation communicates central IL-1 signals regulating bone mass accrual[J]. Proceed Natl Acad Sci US A, 2012, 109: 15455–15460. DOI:10.1073/pnas.1206061109 |
| [18] | Fukuda T, Takeda S, Xu R, et al. Sema3A regulates bone-mass accrual through sensory innervations[J]. Nature, 2013, 497: 490–493. DOI:10.1038/nature12115 |
| [19] | Deng X, Liang LN, Zhu D, et al. Wedelolactone inhibits osteoclastogenesis but enhances osteoblastogenesis through altering different semaphorins production[J]. Int Immunopharmacol, 2018, 60: 41–49. DOI:10.1016/j.intimp.2018.04.037 |
| [20] | Oury F, Khrimian L, Denny CA, et al. Maternal and offspring pools of osteocalcin influence brain develop-ment and functions[J]. Cell, 2013, 155: 228–241. DOI:10.1016/j.cell.2013.08.042 |
| [21] | Mosialou I, Shikhel S, Liu JM, et al. MC4R-dependent suppression of appetite by bone-derived lipocalin 2[J]. Nature, 2017, 543: 385–390. DOI:10.1038/nature21697 |
| [22] | Wise RA, Mcdevitt RA. Drive and reinforcement circuitry in the brain: origins, neurotransmitters, and projection fields[J]. Neuropsychopharmacology, 2018, 43: 680–689. DOI:10.1038/npp.2017.228 |
| [23] | Ende G. Proton magnetic resonance spectroscopy: relevance of glutamate and GABA to neuropsychology[J]. Neuropsychol Rev, 2015: 315–325. |
| [24] | Khrimian L, Obri A, Ramos-Brossier M, et al. Gpr158 mediates osteocalcin's regulation of cognition[J]. Exp Med, 2017, 214: 2859–2873. DOI:10.1084/jem.20171320 |
| [25] | Ketteler M, Block GA, Evenepoel P, et al. Execu-tive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: what's changed and why it matters[J]. Kidney Int, 2017, 92: 26–36. DOI:10.1016/j.kint.2017.04.006 |
| [26] | Mazzaferro S, Pasquali M, Pirrò G, et al. The bone and the kidney[J]. Arch Biochem Biophysics, 2010, 503: 95–102. DOI:10.1016/j.abb.2010.06.028 |
| [27] | Serra Ucer, Srividhya Iyer, Ha-Neui Kim, et al. The effects of aging and sex steroid deficiency on the murine skeleton are independent and mechanistically distinct[J]. J Bone Miner Res, 2017, 32: 560–574. DOI:10.1002/jbmr.3014 |
| [28] | Oury F, Sumara G, Sumara O, et al. Endocrine regulation of male fertility by the skeleton[J]. Cell, 2011, 144: 796–809. DOI:10.1016/j.cell.2011.02.004 |
| [29] | Kirmani S, Atkinson EJ, Rd ML, et al. Relationship of testosterone and osteocalcin levels during growth[J]. J Bone Miner Res, 2011, 26: 2212–2216. DOI:10.1002/jbmr.421 |
| [30] | Barbonetti A, D'Andrea S, Samavat J, et al. Can the positive association of osteocalcin with testosterone be unmasked when the preeminent hypothalamic-pituitary regulation of testosterone production is impaired? The model of spinal cord injury[J]. J Endocrinol Invest, 2018, 42: 1–7. |
| [31] | Kanazawa I. Interaction between bone and glucose metabolism[J]. Endocrine J, 2017, 64: 1043–1053. DOI:10.1507/endocrj.EJ17-0323 |
| [32] | Karsenty G. Convergence between bone and energy homeostases: leptin regulation of bone mass[J]. Cell Metab, 2006, 4: 341–348. DOI:10.1016/j.cmet.2006.10.008 |
| [33] | Ferron M, Wei J, Yoshizawa T, et al. Insulin signal-ing in osteoblasts integrates bone remodeling and energy metabolism[J]. Cell, 2010, 142: 296–308. DOI:10.1016/j.cell.2010.06.003 |
| [34] | Na KL, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton[J]. Cell, 2007, 130: 456–469. DOI:10.1016/j.cell.2007.05.047 |
| [35] | Oldknow KJ, Macrae VE, Farquharson C. The endocrine role of bone: recent and emerging perspectives beyond osteocalcin[J]. J Endocrinol, 2015, 225: R1-19. |
| [36] | Kindblom JM, Ohlsson C, Ljunggren O, et al. Plasma osteocalcin is inversely related to fat mass and plasma glucose in elderly Swedish men[J]. J Bone Miner Res, 2010, 25: 785–791. |
| [37] | Moon SS, Lee YS, Kim SW. Association of nonalcoholic fatty liver disease with low bone mass in postmenopausal women[J]. Endocrine, 2012, 42: 423–429. DOI:10.1007/s12020-012-9639-6 |
| [38] | Chang IC, Chiang TI, Yeh KT, et al. Increased serum osteopontin is a risk factor for osteoporosis in menopausal women[J]. Osteoporos Int, 2010, 21: 1401–1409. DOI:10.1007/s00198-009-1107-7 |
| [39] | Yilmaz Y, Kurt R, Eren F, et al. Serum osteocalcin levels in patients with nonalcoholic fatty liver disease: association with ballooning degeneration[J]. Scandinav J Clin Lab Invest, 2011, 71: 631–636. DOI:10.3109/00365513.2011.604427 |
| [40] | Gupte AA, Sabek OM, Fraga D, et al. Osteocalcin protects against nonalcoholic steatohepatitis in a mouse model of metabolic syndrome[J]. Endocrinology, 2014, 155: 4697–4705. DOI:10.1210/en.2014-1430 |
| [41] | Zhou B, Li H, Xu L, et al. Osteocalcin reverses endoplasmic reticulum stress and improves impaired insulin sensitivity secondary to diet-induced obesity through nuclear factor-ÎB signaling pathway[J]. Endocrinology, 2013, 154: 1055–1068. DOI:10.1210/en.2012-2144 |
| [42] | Karsenty G, Olson EN. Bone and muscle endocrine functions: unexpected paradigms of inter-organ communication[J]. Cell, 2016, 164: 1248–1256. DOI:10.1016/j.cell.2016.02.043 |
| (收稿日期:2018-12-10) |